Definition of periodic table
Miscellanea / / July 04, 2021
By Florencia Ucha, on Jun. 2012
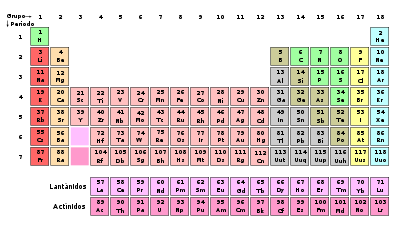 The periodic table to dry, or the periodic table of elements, as it is also called, is a table that classifies, organizes and distributes the different existing chemical elements, being then its primary mission the ordering from the grouping of the elements that make it up, while the atomic mass that they have is the base this classification and ordination.
The periodic table to dry, or the periodic table of elements, as it is also called, is a table that classifies, organizes and distributes the different existing chemical elements, being then its primary mission the ordering from the grouping of the elements that make it up, while the atomic mass that they have is the base this classification and ordination.
Table that orders, classifies and distributes the chemical elements in relation to their atomic numbers in increasing order
The table has a Format from scheme in the form of a table in which all known chemical elements appear, systematically organized according to their atomic numbers in increasing order.
How it is organized
The arrangement is made in 18 vertical columns and by groups of similar elements in terms of properties, given that it attributes the same atomic valence to them.
The groups are namely: the alkali metals, the alkaline earth metals, family from scandium, titanium, vanadium, chromium, magnesium, iron, etc.
On the other hand, it has seven horizontal rows in which the elements that have similar masses are located, although they present different properties.
On the left and in the center of the table are the metals, which are by case the most numerous elements; on the right are nonmetals, with the exception of gases nobles.
At the top of the table you can see a key that has the function of clarifying the meaning of the numbers arranged in the box that corresponds to each of the elements.
And at the bottom of it appear the internal transition elements.
Each symbol is assigned a different color that refers to its state of aggregation, that is, if a temperature environment is a solid, a liquid, or a gas.
Currently this table has a special presence when it comes to bringing knowledge about chemistry, since the study of it is part of the secondary school programs in the area of the aforementioned matter.
Undoubtedly, it is a fundamental and useful instrument when studying chemistry since it allows us to know the similarities between the different chemical elements and understand what could result from the unions between them if produced.
History and scientists who contributed his wisdom to its creation
The Russian chemist Dmitri Ivanovich Mendeleev is regarded as the maker of it, though, the German chemist Julius Lothar von Meyer, contemporary and Mendeleev's rival was also decisive in this regard, creating an ordered table based on the physical properties of atoms.
Subsequently, the Swiss chemist Alfred Werner proposed the current version of the table that presents some modifications with respect to that of Mendeleev.
Therefore it is that the periodic table that we all know today and have learned opportunely in the matter of physical chemistry in the school Secondary is a variant of the one made in 1869 by the Russian chemist, Mendeleev, already mentioned, and by his colleague Meyer; both worked separately and ordered the elements according to the atomic mass available to each one, even leaving places empty in said table, because they anticipated that more elements would continue to appear in the near future, and that is exactly what it happened.
Events that influenced its preparation
It is impossible not to relate the appearance of the periodic table to various issues that were developed in the fields of physics and chemistry, such as ...
The discovery of the elements (copper, gold, lead, silver, carbon, iron, tin, sulfur, mercury, arsenic, tin, among others), the study of the properties that shared these elements and their appropriate classification, the concept of atomic mass, which is the total mass of protons and neutrons present in a single atom when it is not found in movement, and the relationships that were established between the atomic mass and the properties of the elements.
Many elements were already public knowledge since ancient times, although, it should be noted, that from the 18th century the knowledge of new elements was fabulous, especially gases.
Also, by that time Antoine Lavoisier proposes his list of simple substances that expands the knowledge of 33 elements.
In the 19th century, the application of the electric battery in chemical work facilitated the discovery of the alkali metal and alkaline earth elements.
Full Image of the Periodic Table
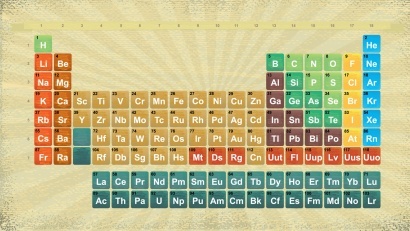
Image: iStock, jelen80
