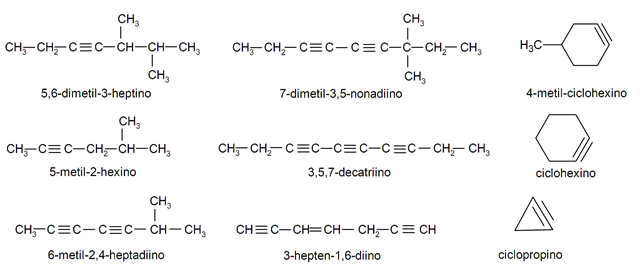20 Examples of Alkynes
Miscellanea / / July 04, 2021
The alkynes or acetylenic hydrocarbons They are hydrocarbons characterized by the presence of a carbon-carbon triple bond. Non-cyclic alkynes respond to the molecular formula CnH2n-2, where n represents the number of carbon atoms. They have a degree of establishment even higher than that of the alkenes. For example: ethyne, propyne, 2-butyne, 3-octyne.
Between the physical properties of alkynes, the fact that they are compounds low polarity, therefore insoluble in water but quite soluble in common organic solvents such as ether, benzene or carbon tetrachloride.
The boiling and melting points of alkynes are very similar to those of alkanes or alkenes of equal carbon number. On the other hand, the effect on these physical properties of the number of carbon atoms and the presence of branches in the chain (which change in the same direction) is more noticeable. That is, the boiling and melting point increases the more carbon atoms the alkyne has.
The simplest alkyne is acetylene, followed by propylene (or propyne) and butyne, which can be 1-butyne (triple bond at the end of the
molecule) or 2-butyne (triple bond in the center of the molecule). These three are gases; those with the highest number of carbon atoms are liquid or solid.Similarly to alkenes, the triple links that characterize alkynes give great chemical reactivity to these substances and make them very prone to undergo addition reactions (hydrogen, halogen, water, etc.) and other However, the three links that join a atom of carbon with another are not equivalent: one of them (which is called the sigma bond) is stronger and acts as the main responsible for the union. When an alkyne is hydrogenated, the resulting compound can become double bonds or just single bonds.
Compounds that have the triple bond at one end of the chain are called terminal alkynes. These compounds are characterized by their marked acidity, in fact, terminal alkynes are the most acidic simple hydrocarbons.
The length between carbon atoms in the bond is 1.20 pm (picometers), even less than that of alkenes (1.34 pm) and that of alkanes (1.54 pm). Single, double and triple carbon-carbon bonds can coexist in the same molecule. When this happens, the hydrocarbon is named as an alkyne and the position of the double bond is marked with the ending "ene", inserting it where appropriate.
Examples of alkynes
Examples of alkynes are shown below:
- ethyne
- tip
- 2-butyne
- 2-pentine
- 1-butyne
- October 3
- cyclobutine
- tert-butyl acetylene
- 6,6-diethyl-4-nonino
- 1-ene-4-hexyne
- 2-nonino
Diagrams of the chemical compounds of these alkynes: