20 Examples of Hydrocarbons
Miscellanea / / July 04, 2021
The hydrocarbons are organic compounds formed exclusively by a structure of hydrogen and carbon atoms, and which are the basis of all the organic chemistry. For example: methane, hexane, phenol, petroleum. The structure of hydrocarbons can be linear or branched, open or closed. In addition, according to their linear and spatial ordering, and their amount of atoms It will depend on whether it is one or the other substance. They can also have atoms other than carbon and hydrogen in their structure, in which case they are called substituted hydrocarbons.
Hydrocarbons are flammable substances and with a wide capacity for industrial transformation, which is why they constitute the basis of world mining extraction, which allows the development of complex materials, caloric and electrical energies, and lighting, among other applications possible. They are also a considerable source of poisoning, as they often give off vapors that are harmful to health.
Hydrocarbons are classified according to several possible criteria:
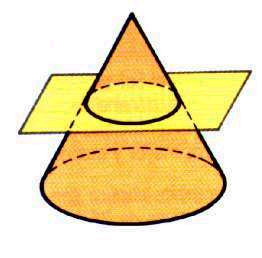
According to its structure:
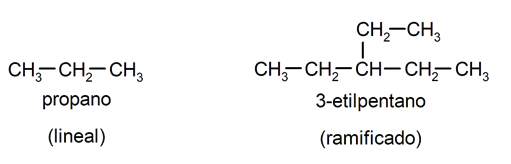 [/ su_list]
[/ su_list]
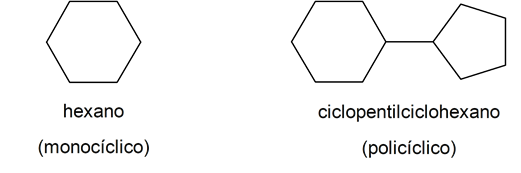
According to the type of bond between its atoms:


Examples of hydrocarbons
- Methane (CH4). It is a gas with a repulsive odor, very flammable, present in the atmosphere of the great gaseous planets and in ours it is the product of the decomposition of the organic material or product of mining activities.
- Ethane (C2H6). It is a highly flammable gas, one of those that constitutes natural gas and is capable of producing frostbite in contact with organic tissues.
- Butane (C4H10). It is a colorless and stable fas, widely used as a high pressure (liquid) fuel in the domestic context.
- Propane (C3H8). It's a gas, colorless and odorless, endowed with high explosiveness and narcotic properties when exposure to high concentrations occurs.
- Pentane (C5H12). It is an alkane that is normally in a liquid state. It is used as a solvent and as a source of energy, given its high safety and low cost.
- Benzene (C6H6). It's a liquid colorless with a sweet aroma, highly flammable and also highly carcinogenic, it is among the most widely produced industrial products today. It is used in the manufacture of rubbers, detergents, pesticides, medicines, plastics, resins and in the refining of petroleum.
- Hexane (C6H14). It is used as a solvent in some paints and adhesives, as well as in obtaining pomace oil. Its use, however, is restricted because it is an addictive neurotoxic.
- Heptane (C7H16). It is a liquid that, under pressure and temperature environmental, it is highly flammable and explosive. It is used in industry of the fuels as the zero point of octane, and as a working base in pharmaceuticals.
- Octane (C8H18). It is the 100th point on the gasoline octane scale, opposite to heptane, and has a long list of isomers for industrial use.
- 1-Hexene (C6H12). Classified in the industry as a superior olefin and alpha-olefin, it is a colorless liquid essential in obtaining polyethylene and certain aldehydes.
- Ethylene (C2H4). The most widely used organic compound in the world is at the same time a natural hormone of the plants and an industrial compound necessary for the manufacture of plastic. It is usually obtained from the dehydrogenation of ethane.
- Acetylene (C2H2). It is a colorless gas, lighter than air and highly flammable, which produces a flame capable of reaching 3000 ° C, one of the highest temperatures manageable by man. It is used as a source of lighting and heat in various industries and applications.
- Trichlorethylene (C2HCL3). It is a colorless, flammable liquid, with a sweet smell and taste, highly carcinogenic and toxic, capable of interrupting the cardiac, respiratory and hepatic cycles. It is a powerful solvent for industrial use that does not exist in nature.
- Trinitrotoluene (C7H5N3OR6). Known as TNT, it is a pale yellow, crystalline, highly explosive compound. Does not react with metals nor does it absorb water, so it has a long life and is widely used as part of military and industrial bombs and explosives.
- Phenol (C6H6OR). Also known as acid carbolic or phenyl or phenylhydroxide, it is solid in its pure, crystalline and white or colorless form. It is used to obtain resins, nylon and as a disinfectant or part of various medical preparations.
- Tar. It is a complex mixture of organic compounds whose formula varies according to the nature of its production and its temperature and other variables. Is a liquid substance, bituminous, slimy and dark, with a strong odor and many applications, from psoriasis treatment to road paving.
- Kerosene. It is a common fuel, not very clean and obtained by petroleum distillation natural. It is composed of a mixture of hydrocarbons in a transparent and yellowish liquid, insoluble to water, used for lighting and surface cleaning purposes, as well as pesticide and lubricant motor.
- Gasoline. Obtained from petroleum by direct or fractional distillation, this mixture of hundreds of hydrocarbons is used in combustion engines internal fuel as the cleanest, most efficient and popular fuel known, especially after it was separated from lead in the early 1900s. 2000.
-
Petroleum. It is the most important mixture of hydrocarbons known in industrial terms. From oil it is possible to synthesize many other and diverse types of substances. It is produced underground from organic matter accumulated in geological traps and subjected to extremely high pressure. It is of fossil origin, a viscous and dense black liquid, whose world reserves are Non-renewable, but it constitutes the main input for the automotive, electrical, chemical and materials industries.

Follow with: