20 Examples of Alkenes
Miscellanea / / July 04, 2021
The alkenes They are compounds containing carbon-carbon double bonds. When these compounds have open chain structures they respond to the molecular formula CnH2n (where n is the number of carbon atoms). Alkenes are also called olefins and correspond to the group of hydrocarbons unsaturated. They are obtained mainly as part of the oil cracking process and by dehydrogenation of alkanes. For example: ethene, propene, cyclohexene.
They are organic compounds that can be short, medium or long chain; there are also cyclic alkenes or cycloalkenes. By having the carbon-carbon double bond, alkenes have fewer hydrogens than alkanes corresponding with equal number of atoms carbon.
How are alkenes named?
To name the alkenes, the carbon chain that contains the greatest amount of carbon atoms and that also contains the double bond. If that chain has several double bonds, they are named looking for the smallest possible combination of the positions of those double bonds.
The position of the double bond It is indicated by inserting before the suffix -no the Latin prefix that indicates the number of the carbon where the double bond begins (di (2), tri (3), tetra (4), penta (5), octa (8), etc. ). Substituents (usually chlorine, bromine, ethyl, methyl, etc.) are named as prefixes (at the beginning of the name), detailed and in alphabetical order, they are also named looking for the smallest possible combination of their positions in the chain. For example:
1-butene / 1,2-butadiene / 5-chloro-1-pentene / 4-pentenyl chloride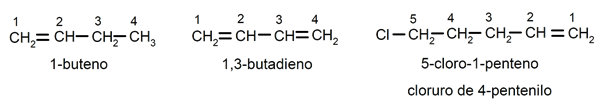
Given how complex the chemical name established according to the IUPAC criteriaMany naturally occurring organic alkenes have fancy names, often related to their natural source. For example: limonene / 1-methyl-4- (1-methylethenyl) -cyclohexene / geraniol / 3,7-dimethyl-2,6-octadien-1-ol
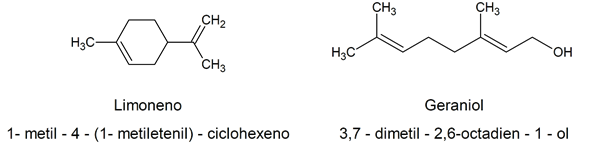
Alkenes of up to four carbons are gases at room temperature, those with 4 to 18 carbons They are liquids and the longest are solid. They solubilize in solvents organic such as ether or alcohol and are slightly more dense than the corresponding alkanes (that is, with the same number of carbon atoms).
The point of fusion Y boiling of alkenes, as in alkanes, increases the longer the carbon chain.
On the other hand, due to the voltage generated by the double bond, the distance between the carbon atoms involved in the double bond in the alkene is 1.34 pm (picometers), while the distance of the single bond in the corresponding alkane is 1.54 p.m.
They present a chemical reactivity much higher than alkanes, precisely because they have those double bonds that have a density electronics that are high and can break down and allow the addition of other atoms, often hydrogen or halogens. They can also experience oxidation and polymerization.
Alkenes usually present cis-trans isomerism or stereoisomerism, since the carbon atoms connected by the double bond cannot rotate and this causes the substituents to be located on the same side of the double bond or on opposite sides. For example: trans-2-butene / cis-2-butene
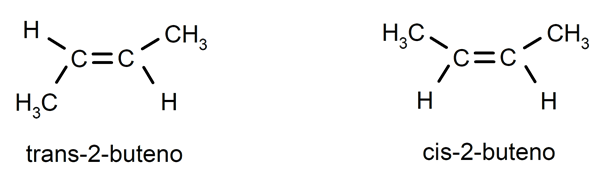
Alkenes with two double bonds are called dienes, and those with more than two double bonds are generally called polyenes.
In the plant world, alkenes are quite abundant and have physiological roles very significant, such as the regulation of the fruit ripening process or the filtration of certain solar radiation.
The chemical structure of organic alkenes is usually quite complex and includes carbon chains and rings. Some fruits (such as carrots or tomatoes) and some crustaceans (like crabs) produce significant amounts of beta carotene, an important alkene that is a precursor to vitamin A.
Examples of alkenes
- ethene
- 2-methylpropene
- propene
- 2,3-butadiene
- myrcene
- limonene
- geraniol
- lycopene
- lanosterol
- mentofuran
- tetrafluoroethylene
- 1,3,5,7-cyclooctatetraene
- 5-bromo-3-methyl-3-hexene
- 3,5-diethyl-4-propyl-2,5-dimethyl-2-heptene
- 7,7,8-trimethyl-3,5-nonadiene
- 3,3-diethyl-1,4-hexadiene
- cyclohexene
- 1-chloro-2-methylprop-1-ene
- 3-bromo-5-methyl-cyclopentene
- 1-methyl-cyclohexene
Diagrams of the chemical compounds of these alkenes:



