20 Examples of Alkanes
Miscellanea / / July 04, 2021
The alkanes they are a kind of hydrocarbons in which a variable number of carbon atoms are joined together by single bonds, like a skeleton, and each carbon atom is in turn attached to hydrogen atoms, which may eventually be replaced by others atoms or functional groups. For example: chloroform, methane, octane.
The molecular formula of the open linear chain alkanes is CnH2n + 2, where C represents carbon, H represents hydrogen and n represents the number of carbon atoms. Alkanes are saturated hydrocarbons, which means they do not have double or triple bonds. To name them, you use the suffix "-Ano" after naming the carbon chain using the prefix corresponding to the number of carbon atoms (et- (2), pro- (3), but- (4), pen- (5), hex- (6), hep- (7), etc).
It can serve you:
Classification of alkanes
Within the alkanes they are usually recognized two large groups: open chain (also called acyclic) and closed chain (or cyclical).
When the open chain compounds They do not present any substitution of the hydrogens that accompany each carbon atom, they are called linear alkanes: these are the simplest alkanes. When they present a substitution of any of their hydrogens by one or more carbon chains, they are called branched alkanes. The most common substituents are ethyl groups (CH
3CH2-) and methyl (CH3-).For their part, there are compounds with a single cycle in the molecule (monocyclic) and others with several (polycyclic). The cyclic alkanes they can be homocyclic (they are formed with the exclusive intervention of carbon atoms) or heterocyclic (in which other atoms participate, for example, oxygen or sulfur).
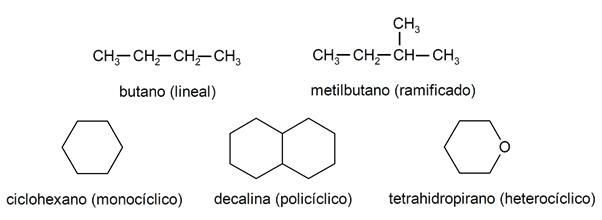
Physical properties of alkanes
In general, physical properties of alkanes are conditioned by molecular mass (in turn linked to the length of the carbon chain). Those with the lowest number of carbons are gaseous to temperature environment, those ranging from 5 to 18 carbon atoms are liquids, and above this number are solid (similar to wax).
Being less dense than water, they tend to float on it. In general, alkanes are insoluble in water and soluble in organic solvents.
The point of boiling and of fusion of alkanes depends on their molecular mass, that is, on the length of the carbon chain, although they also depend many times on the spatial arrangement of the atoms. Linear and cyclic alkanes have higher boiling points than branched ones.
Chemical properties of alkanes
Alkanes are characterized by being chemical compounds of very poor reactivity, which is why they are also known as "paraffins" (in Latin, parum affinis means "low affinity"). They are compounds that have a very high activation energy when they are involved in chemical reactions. The most important reaction that alkanes can undergo is combustion generating, in the presence of oxygen, heat, carbon dioxide and water.
Alkanes are the basis for an important variety of reactions associated with industrial processes very important, being the most traditional fuels. They also appear as end products of biological processes such as methanogenic fermentation carried out by some microorganisms.
Examples of alkanes
Some examples of alkanes (including some well-known linear and branched ones) are:
- Chloroform (fancy name of the trichloromethane; CHCl3). The vapors of this substance used to be used as anesthetics. It has been discontinued for this purpose because it was found to damage organs important, such as the liver or kidneys. Its use today is primarily as a solvent or coolant.
- Methane (CH4). It is the simplest alkane of all: it is made up of just one carbon atom and four hydrogen atoms. It is a gas that occurs naturally by the decomposition of different organic substrates and is the main component of natural gas. In recent times it has been recognized as one of the gases that contributes the most to the so-called greenhouse effect.
- Octane (C8H18). It is the eight-carbon alkane and is of great importance since it determines the final quality of the naphtha, which is a mix of various hydrocarbons. This quality is measured by the octane or octane number of the fuel, which takes as a reference a low-detonating one (index 100) and a highly detonating one (index 0).
- Hexane (C6H14). It is an important solvent, its inhalation should be avoided since it is very toxic.
- Butane (C4H10). Along with propane (C3H8), make up the so-called liquefied petroleum gases (LPG), which are formed in gas bags during the oil extraction process. Currently, the replacement of gasoline or diesel by LPG as fuel is being promoted since it is of a more environmentally friendly hydrocarbon (it emits only carbon dioxide and water during its combustion).
- Icosano (C20H42). It is the twenty-carbon alkane (the prefix 'ico' means twenty)
- Cyclopropane (C3H6). Formerly it was used as an anesthetic
- n-heptane (C7H16). It is the one that is taken as a reference for the zero point of the gasoline octane scale, which would be the least desirable, since it burns explosively. It is obtained from the resin of certain plants.
- 3-ethyl-2,3-dimethylpentane (C9H20)
- 2-methylbutane (C5H12)
- 3-chloro-4-n-propylheptane (C10H21Cl)
- 3,4,6-trimethylheptane (C10H22)
- 1-bromo-2-phenylethane (C8H9Br)
- 3-ethyl-4-methylhexane (C9H18)
- 5-isopropyl-3-methylnonane (C13H28)
- Cuban (C8H8)
- 1-bromopropane (C3H7Br)
- 3-methyl-5-n-propyloctane (C12H26)
- 5-n-butyl-4,7-diethyldecane (C18H28)
- 3,3-dimethyldecane (C12H26)

