Concept in Definition ABC
Miscellanea / / November 09, 2021
Conceptual definition
Distillation is a widely used separation method in industry that is based on the evaporation temperature difference of two or more substances that make up a mixture. Once the substance to be separated is evaporated, it is collected or recovered through its consequent condensation.

Chemical engineer
This practice can be carried out both at the laboratory level and at the industrial level, in the following scheme a distillation process is observed at the level scale laboratory, where several components are recognized, firstly, a reservoir where the mixture to be distilled is stored, at its top there is a thermometer to register the temperature at which distillation of the desired substance occurs.
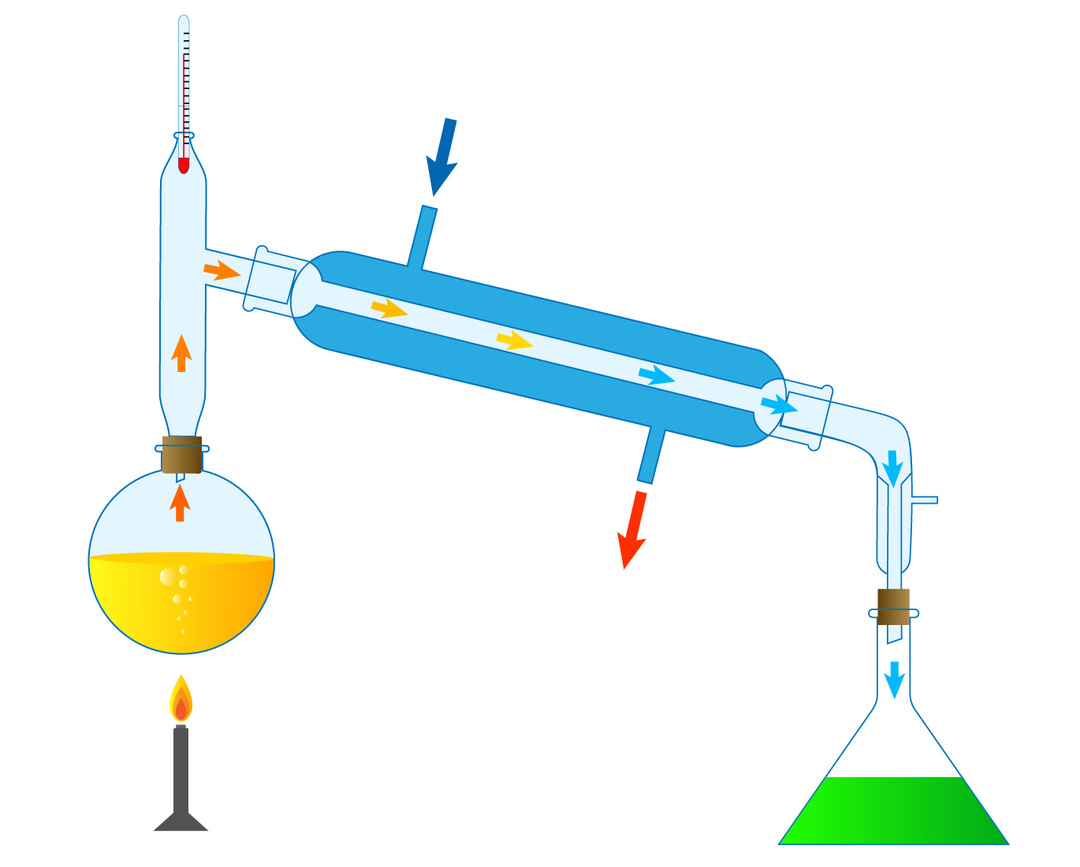
As the mixture is heated by the burner located under the container, the most volatile component will be the one that passes as steam to the condenser, where by a drop in temperature, thanks to the passage of refrigerant through the external area of the condenser. The condensate or distillate will be collected in the
flask positioned to the right of the image. In this way, we are able to separate a mixture by means of a physical change.For example, if we wanted to separate table salt dissolved in water, we could carry out the experience, where the water will evaporate and then condense collecting it in a flask while the salt will remain in the reservoir initial.
Types of distillation
Distillation is widely used in the industry of food and hydrocarbons as a method of separation. In the oil and gas industry, a distillation known as fractional distillation is carried out. By incorporating as a current of feeding a mixture of hydrocarbons in a distillation tower, due to differences in their boiling points we can obtain the different components of the mixture at different heights of the tower.
At the top of the tower, the most volatile gases will be obtained (compounds that evaporate at a lower temperature) while in the background the components with a higher molar mass, since they have a higher boiling point. In this process, the different boiling temperatures determine different heights of the tower, where various outlets are connected to collect the different compounds.
In general, in the case of hydrocarbons, gases will be obtained at the top, then gasoline, kerosene, oil fuel, lubricating oils and, finally, in the background, asphalt.
Now, a problem faced by the hydrocarbon industry is the market demand, there is a greater demand for gasoline than for the other compounds in the mixture, for In order to meet market requirements, the hydrocarbons at the bottom of the tower are subjected to other processes that will convert them into more hydrocarbons. tools.
Another type of practice used is steam distillation, in which we can separate substances that are insoluble in water but which, in turn, are volatile and are combined with products not volatile. This technique is used in compounds that decompose prior to their evaporation or whose boiling point is higher than 100ºC. Such is the case of essential oils, for example, essential oils of citrus or spices are extracted under this method to later use this pure essence in different applications such as perfumes or perfumes.
When carrying out this distillation, the compound that we want to extract its essence (for example, lemon peels) is heated with a sufficient amount of water. As the water begins to evaporate, the steam will carry away the volatile and water-insoluble compounds that will be collected in a collecting flask after passing through a condenser, while in another flask non-volatile and / or soluble compounds in Water.
Also, this method is used in the hydrocarbon industry, since as shown in the example, the essential compounds are also organic compounds, like hydrocarbons and many of them tend to decompose when subjected to high temperatures in a sustained way, making it an attractive method for their separation.
In the case of "distilled water", which is available over the counter to be placed, for example, in car glass cleaners, in general, it is water from quality "Demineralized" that is not necessarily obtained by a distillation method. The distillation of water seeks to remove some ionic species that give it the character of hardness, as well as dissolved gases and organic compounds. Many times, the "distilled" water that we can buy is obtained by more economical and efficient methods than distillation and therefore, it is It is correct to call it for its "demineralized" quality, which shows that it is avid for certain minerals according to the purification method. employee.
Illustration: Saint
Topics in Distillation