100 Examples of Chemical Elements
Miscellanea / / November 13, 2021
The chemical elements are a type of matter that by no procedure or chemical reaction they can be reduced or decomposed into other simpler elements. For this reason, it can be said that an element is all matter made by atoms of the same and unique class, that is, atoms that have the same atomic number (equal number of protons), although they have different atomic mass. For example: sulfur, boron, chromium, tin.
The first definition of a chemical element was introduced by Lavoisier in the Traite Élémentaire de Chimie, in 1789. Back in the 18th century, Lavoisier subdivided simple substances into four groups:
Periodic table of elements
Today they know each other 118 chemical elements. They are all gathered, classified and organized according to many of their properties in a graphical scheme known as the Periodic Table of the Elements, which was originally created by the Russian chemist Dimitri Mendeleyev in 1869. The Periodic Table is composed of 18 groups (columns) and 7 periods (rows), in which the chemical elements are located.
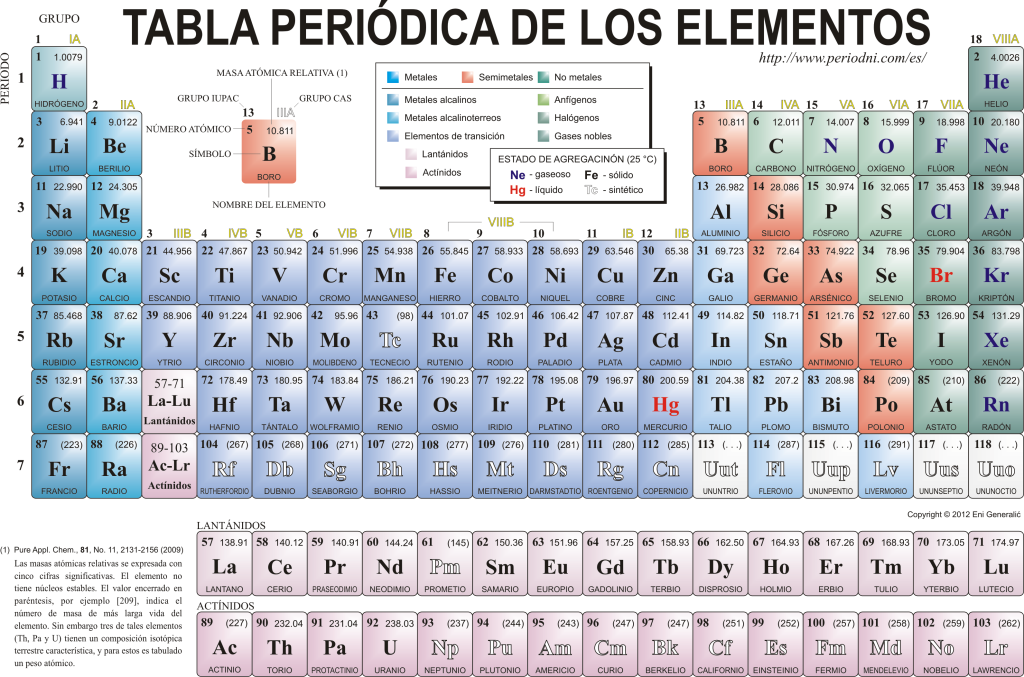
The main groups that can be found in this table are:
The alkali metals (Group 1), the alkaline earth metals (Group 2), the scandium family, which includes the earths and actinides (Group 3), the titanium family (Group 4), the vanadium family (Group 5), the chromium family (Group 6), the manganese family (Group 7), the iron family (Group 8), the cobalt family (Group 9), the nickel family (Group 10), the copper family (Group 11), the zinc family (Group 12), earth elements (Group 13), carbonid elements, in this group is carbon, which constitutes the basis of life on Earth (Group 14), nitrogen-forming elements (Group 15), amphigenetic elements, includes oxygen, a fundamental element for the breathing of the living beings (Group 16), halogen elements (Group 17) and noble gases (Group 18).
Many of these elements have stable or radioactive isotopes, that is, the same chemical element, for example, hydrogen (H), can have several isotopes (1H, 2H, 3H). This means that each isotope atom has the same number of protons (which implies that it belongs to the same chemical element) and a different number of neutrons.
Some isotopes are not stable (radioactive), that is, disintegrate in a certain time, emitting certain particles (neutrons, photons, alpha particles, among others) and generate other stable or radioactive isotopes. Chemical elements have properties such as the point of boiling and that of fusion, electronegativity, density and ionic radius, among others. These properties are important because they allow us to predict its behavior, reactivity, etc.
How are the elements presented in the Periodic Table?
Each chemical element is characterized in the Periodic table using certain symbology. In the first place, in the center of each square its universal symbol, which consists of one or two letters (by convention, if there are two letters, the first is written in uppercase and the next in lowercase).
Above and to the left appears in small typeface su atomic number, which is the one that indicates the amount of protons that this element has. Below the element symbol its name appears and above, on the left, its name appears. atomic mass relative. In addition, the color in which the element symbol is represented signifies its state of aggregation (according to the representation used in each Periodic Table).
The different elements present atomic radii variables and, as the number of protons in the nucleus increases, the greater the attraction that the nucleus exerts on the electrons, so the atomic radius tends to decrease. When the atomic radius is small, the electrons at the outermost level of the cloud are very attracted to the nucleus, so they do not give up easily. The opposite happens with elements with high atomic radii: they give up their outer electrons easily.
Thus, the atomic radius increases from top to bottom when we go through the groups of the Periodic Table and decreases from left to right as we go through their periods.
Examples of chemical elements
| Chemical element | Symbol |
| Actinium | Ac |
| Aluminum | To the |
| Americium | A.M |
| Antimony | Sb |
| Argon | Ar |
| Arsenic | Ace |
| Astat | At |
| Sulfur | S |
| Barium | Ba |
| Beryllium | Be |
| Berkelium | Bk |
| Bismuth | Bi |
| Bohrio | Bh |
| Boron | B |
| Bromine | Br |
| Cadmium | CD |
| Calcium | AC |
| Californium | Cf |
| Carbon | C |
| Cerium | EC |
| Cesium | Cs |
| Chlorine | Cl |
| Cobalt | Co |
| Copper | Cu |
| Chrome | Cr |
| Curium | Cm |
| Darmstadio | Ds |
| Dysprosium | Dy |
| Dubnium | Db |
| Einsteinium | It is |
| Erbium | Er |
| Scandium | Sc |
| Tin | Sn |
| Strontium | Mr |
| Europium | Eu |
| Fermium | Fm |
| Fluorine | F |
| Match | P |
| Francius | Fr |
| Gadolinium | Gd |
| Gallium | Ga |
| Germanium | Ge |
| Hafnium | Hf |
| Hassio | Hs |
| Helium | I have |
| Hydrogen | H |
| Iron | Faith |
| Holmium | Ho |
| Indian | In |
| Iodine | I |
| Iridium | To go |
| Ytterbium | Yb |
| Yttrium | Y |
| Krypton | Kr |
| Lanthanum | The |
| Lawrencio | Lr |
| Lithium | Li |
| Lutetium | Mon |
| Magnesium | Mg |
| Manganese | Mn |
| Meitnerius | Mt |
| Mendelevium | Md |
| Mercury | Hg |
| Molybdenum | Mo |
| Neodymium | Na |
| Neon | Ne |
| Neptunium | Np |
| Niobium | Nb |
| Nickel | Neither |
| Nitrogen | N |
| Nobelio | Not |
| Gold | Au |
| Osmium | You |
| Oxygen | OR |
| Palladium | P.S |
| Silver | Ag |
| Platinum | Pt |
| Lead | Pb |
| Plutonium | Pu |
| Polonium | Po |
| Potassium | K |
| Praseodymium | Pr |
| Promise | P.m |
| Protactinium | Pa |
| Radio | Ra |
| Radon | Rn |
| Rhenium | Re |
| Rhodium | Rh |
| Rubidium | Rb |
| Ruthenium | Ru |
| Rutherfordio | Rf |
| Samarium | Ye |
| Seaborgio | Sg |
| Selenium | I know |
| Silicon | Yes |
| Sodium | Na |
| Thallium | Tl |
| Tantalum | Ta |
| Technetium | Tc |
| Tellurium | Tea |
| Terbium | Tb |
| Titanium | You |
| Thorium | Th |
| Thulium | Tm |
| Copernicus | Cn |
| Livermorio | Lv |
| Roentgenio | Rg |
| Ununoctium | Uuo |
| Ununpentium | Uup |
| Flerovio | Fl |
| Ununseptio | Uus |
| Ununtrium | Uut |
| Uranium | OR |
| Vanadium | V |
| Tungsten | W |
| Xenon | Xe |
| Zinc | Zn |
| Zirconium | Zr |

Follow with:



