Example of Organic and Inorganic Molecules
Chemistry / / November 13, 2021
The General chemistry it is the science that studies all types of matter that exist, and their internal changes having contact between different types of this.
The Organic chemistry It is the part of General Chemistry destined to study the matter whose main constituent is the Carbon element, So what it is part of living organisms.
The Inorganic chemistry It is the part of General Chemistry that is in charge of studying the so-called "mineral matter", which is part of the I do not live environment.
The Molecule is the union of various atoms of different Elements chemicals to form new substances, with their particular properties.
In General Chemistry, Elements Are the pure substances which are formed by atoms of a single type. The Elements are classified in the Periodic Table of Chemical Elements.
Just like him Atom is the fundamental unit of the Elements, the Molecule is the main unit of Compounds, which are substances that have a characteristic chemical behavior.
The Compounds may be formed as a consequence of
natural phenomena, or be created in labs or in Industrial plants, so molecules are present everywhere. The molecules are found in minerals, in tree leaves, in food, in medicines, in the water we drink, in the air we breathe, and even in the pollution of the environment.General Chemistry is mainly divided into Inorganic chemistry Y Chemicalto Orgnica, so Molecules can also be classified into Inorganic and Organic.
Inorganic Molecules
In Inorganic Chemistry, molecules are mostly formed by the combination of atoms of positive valences with others of negative valences, in ionic bonds. These bonds are formed mainly by the electromagnetic forces between the atoms, generated by the presence of the valence electrons.
Thus all ionic compounds arise, such as Salts, Oxisalts, Acids, Oxyacids, Oxides and Hydroxides.
Inorganic Molecules as Electrolytes
The main property of ionic molecules is that when they come into contact with Water H2OR, they are separated into its two parts: positive and negative. These two parts, electrically charged atoms or groups of atoms, are dispersed in the water. To the inorganic substance capable of thus separating in water, it is called Electrolyte.
The Solution formed by Water and positively and negatively charged particles is called "Electrolytic Solution". This type of solution has the ability to conduct electrical currents, which is why it is used in electrochemical cells, such as car batteries.
Inorganic Acid and Alkaline Molecules
In the case of inorganic molecules such as Acids, the Oxyacids and the Hydroxides, at the same time that they separate into a positive and negative part, they contribute to the Solution a property called Hydrogen Potential, measured as the negative logarithm of hydrogen ion concentration.
The Hydrogen potential (pH) determines how much the Solution is Acidic. On the pH scale, which ranges from a value of 1 for maximum acidity to 14, which is complete alkalinity or basicity, the acid character ranges from values 1 to 6, and the alkaline is between 8 and 14. 7 represents neutral pH; neither acidic nor basic. The result of the negative logarithm of the H + Concentration will tell us where we are on the scale.
Examples of Acids:
Hydrochloric Acid: HCl: H+ + Cl-
Hydrobromic Acid: HBr: H+ + Br-
Sulfhydric Acid: H2S: 2H+ + S-2
Cyanhydric Acid: HCN: H+ + CN-
Hydrochloric Acid: HI: H+ + I-
Examples of Oxyacids:
Sulfuric Acid: H2SW4: 2H+ + SO4-2
Carbonic Acid: H2CO3: 2H+ + CO3-2
Nitric Acid: HNO3: H+ + NO3-
Phosphoric Acid: H3PO4: 3H+ + PO4-3
Perchloric Acid: HClO4: H+ + ClO4-
Examples of Hydroxides:
Sodium Hydroxide: NaOH: Na+ + OH-
Calcium Hydroxide: Ca (OH)2: Ca+ + 2OH-
Ammonium Hydroxide: NH4OH: NH4+ + OH-
Potassium Hydroxide: KOH: K+ + OH-
Magnesium Hydroxide: Mg (OH)2: Mg+ + 2OH-
Inorganic Molecules in Chemical Reactions
When inorganic molecules participate in a chemical reaction, there can be four basic and simple reaction mechanisms: Synthesis, Decomposition, Simple Substitution and Double Substitution. Here's an example of each:
Synthesis
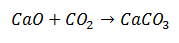
A Synthesis reaction is one in which two molecules come together in a final product consisting of a single molecule. In the example it is the case of Calcium Oxide combining with Carbon Dioxide to form a Calcium Carbonate molecule.
Decomposition:

A Decomposition reaction is one in which an initial molecule separates into two new stable molecules. Such is the case of Calcium Hydroxide, separating into a molecule of Calcium Oxide and another of Water.
Simple Substitution:

In a Simple Substitution Reaction, an atom of an element is exchanged with one of the atoms of a molecule. Such is the case of metallic Zinc, placing itself in the place of Hydrogen in Hydrogen Chloride, releasing it and forming Zinc Chloride molecules.
Double Substitution:
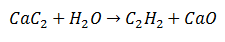
In a Double Substitution Reaction, certain atoms of two initial molecules are exchanged, to generate two different molecules as products. Such is the case of Calcium Carbide, which undergoes the release of Carbon, which will combine with Hydrogen from Water to form Acetylene. Calcium will bind to oxygen to form Calcium Oxide as a second product.
Organic Molecules
Organic Chemistry is Carbon Chemistry, which means that all Organic Molecules will have the presence of this element, in different structural arrangements.
Organic molecules are characterized by constant presence of Covalent Bonds. Covalent Bonds with those in which two atoms join together to share their valence electrons and thus complete their octets mutually.
This is the case with Carbon, which binds to other atoms of the same element. Chains of very varied lengths are formed, from two to sixty carbon atoms, and even these chains they branch with other chains with the same variety of lengths, achieving an immense diversity of molecules organic.
Ionic bonds are also present, but these occur in intermediate steps of long reaction mechanisms in which a desired molecule is formed.
The simplest Organic Molecules include Carbon and Hydrogen. The latter completes the carbon valence that requires it.
In Organic Chemistry, Molecules can be linear or aliphatic, branched, cyclic, and aromatic.
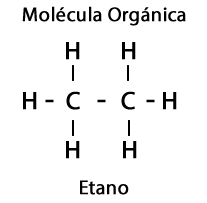
Furthermore, in the Organic Molecules the elements Oxygen, Nitrogen, Sulfur and Phosphorus are involved, which originates an impressive diversity of Functional Groups for the molecules.
Functional Groups in Organic Molecules
The Functional Groups are groups of two or more atoms that, when joining a Carbon-Hydrogen chain, form different chemical species, with a particular behavior. Next, the seven main types of Organic Molecules are listed, with their respective functional groups. The letter "R" is used to denote the Carbon-Hydrogen chain.
Alkyl Halides - Form: R-X / Functional Group: A Halogen element (Chlorine, Bromine, Iodine)

Alcohols - Form: R-OH / Functional Group: -OH or Hydroxyl.
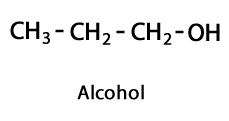
Aldehydes - Form: R-CHO / Functional Group: -CHO, which always goes to the end of the chain.
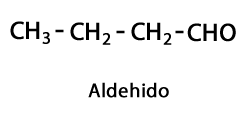
Ketones - Form: R-CO-R / Functional Group: -CO- or Carboxy, always in the middle Carbon of the chain.
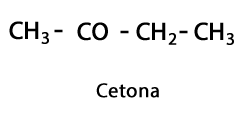
Organic acids - Form: R-COOH / Functional Group: -COOH or Carboxyl, always at the end of the chain.
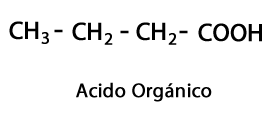
Acid Esters - Form: R-COO-R / Functional Group: -COO-, is the result of joining an acid chain with another Carbon-Hydrogen chain.
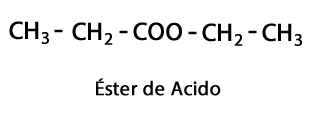
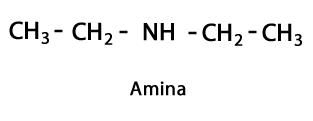
Amines - Form: R-NH2, R-NH-R, R-N-2R / Functional Group: -NH2, -NH-, -N = or Amino, which is a Nitrogen supplemented with Hydrogen in places where there is no Carbon-Hydrogen chain. As stated, it can go at the end of the chain, or in the middle. The Nitrogen atom can be accompanied by one, two or three organic chains to form a final molecule. Amines can be considered organic derivatives of ammonia NH3.
Organic Molecules in Chemical Reactions
Organic molecules, the longer their Carbon-Hydrogen chains, the more sites or atoms available to participate in a chemical reaction.
Most often, elements or chains are added to one of the carbons present, or a part of the main chain is detached to generate a different organic compound apart.
As such reactions are slow, Catalysts are used, which are chemical agents to accelerate the reactions. In some cases, the Catalyst is a fine mesh of Platinum metal.
Examples of Inorganic Molecules
Sodium Chloride NaCl
Potassium Chloride KCl
Ammonium Chloride NH4Cl
Sodium Nitrate NaNO3
Potassium Nitrate KNO3
Ammonium Nitrate NH4NOT3
Sulfuric Acid H2SW4
Phosphoric Acid H3PO4
Phosphorous Acid H3PO3
Hydrochloric Acid HCl
Iodhydric acid HI
Sodium Hydroxide NaOH
Potassium Hydroxide KOH
Ammonium Hydroxide NH4Oh
Calcium Hydroxide Ca (OH)2
Magnesium Hydroxide Mg (OH)2
Ferrous Hydroxide Fe (OH)2
Ferric Hydroxide Fe (OH)3
Iron Sulfide FeS
Ferrous Sulfate FeSO4
Ferric Sulfate Fe2(SW4)3
Examples of organic molecules
Glucose C6H12OR6
Methane CH4
Ethane C2H6
Acetylene C2H2
Propane C3H8
Butane C4H10
Ethanol C2H6OR
Sucrose C12H22OR11
Methanol CH4OR
Glycerol C3H8OR3

