What is CO₂ Corrosion and how is it defined?
Miscellanea / / August 07, 2022
CO2 corrosion is a corrosive phenomenon produced by the presence of gaseous CO2 that is dissolves in the aqueous phase and can generate generalized or localized corrosion, mainly in steels to carbon.






Chemical engineer
Given that of all the failures that occur during the operation of equipment and pipelines that transport natural gas or oil, 33% of them result from corrosive phenomena, this type of corrosion plays a role fundamental. It is known that 28% of these are caused by "sweet" corrosion by CO2, while 18% of failures derive from "acid" corrosion by H2S.
Internal corrosion (on the inner surface) is generally due to the presence of water in conjunction with you go out, carbon dioxide (CO2) and hydrogen sulfide (H2S). That is why carbon dioxide is a corrosive dissolved gas, whose solubility depends on factors such as pressure and temperature of operation. If CO2 comes into contact with the water in the cooling system production, this will be affected since with partial pressures as low as 3 psi, it can result in a thinning agent.
When the CO2, present in the transported fluid, reacts with the water by dissolving, it forms carbonic acid, which interacts with iron (main component of carbon steel) giving rise to a global reaction that generates hydrogen and ions. Furthermore, CO2 can react with iron to form ferrous carbonate (FeCO3).
In the presence of carbonic acid, iron reacts forming said carbonate and precipitating. Therefore, this type of corrosion is easily identifiable based on its morphology of damage and corrosive products found, such as iron carbonates and iron oxides. The reactions involved are as follows:
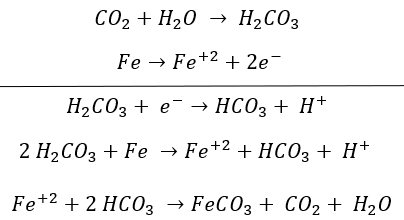
As we mentioned before, the solubility of carbon dioxide plays a fundamental role, since as it increases, there will be more gas dissolved in the aqueous phase. This solubility, as in most of the gases, increases with increasing total pressure and decreasing temperature. Thus, the severity of the damage that occurs depends strongly on these factors, as the concentration of CO2 in the aqueous phase increases. When carbonic acid is produced, the pH of the solution resulting is diminished, this is also a factor to consider when evaluating its corrosive rate and the damage generated.
API 571 determines that the materials most affected by this type of corrosion are: carbon steels and low alloy steels. Whereas, an increase in the Chromium content in the composition of the steel greater than 12%, type 410 SS, reaches a greater endurance. Likewise, the 300 series austenitic stainless steel is also considered to be resistant to CO2 corrosion.
Corrosion by CO2 or sweet corrosion manifests itself in different ways depending on the unit and the equipment with which it is working. Likewise, this damage morphology can vary depending on the interaction with other corrosive agents in the environment such as hydrogen sulfide, oxygen or even chlorides, which accelerate corrosion reactions. The presence of chloride ions is known to decrease the stability of the protective layer. formed by both the precipitated carbonate (FeCO3) and that formed by magnetite (iron oxide, Fe3O4). Therefore, as the chloride concentration increases, corrosive phenomena will be more likely.
In general, a generalized or localized attack can be seen. When this damage is localized in certain areas that are most affected, pitting can be identified (in flow areas tight or semi-tight), "table" type attacks (of the flat type) or even "pits" in areas of high speeds of flow. That is, the morphology is also dependent on many parameters, such as those already mentioned and even the presence or absence of particulate material.
To prevent this type of mild corrosion, corrosion inhibitors are normally used, which form a kind of film or "film". surface protector that act as a "barrier" and even other types of inhibitors that can neutralize the acidity produced by the gas dissolved. Eventually, it is also decided to use materials that are more resistant to this type of corrosion.
References
Asrar, N., MacKay, B., Birketveit, Ø., Stipanicev, M., Jackson, J., Jenkins, A.,... & Vittonato, J. (2016). Corrosion: The longest fight. Oilfield Review, 28(2), 36-51.American Petroleum Institute (Wash.). (2011). Damage Mechanisms Affecting Fixed Equipment in the Refining Industry: API Recommended Practice 571.

