What is the Rankine cycle, and how is it defined?
Inhibition String Theory / / April 02, 2023

Industrial Engineer, MSc in Physics, and EdD
Ideal thermodynamic power cycle, whose purpose is to obtain useful work from a heat source. Its efficiency is limited by the equivalent Carnot cycle that operates between the same temperature ranges and that obeys the second law of thermodynamics. Its name refers to the physicist, engineer and educator William John Macguorn Rankine (1820-1872), who developed this model where he was born, Scotland.
The Rankines cycle is of great importance, since this model is used as a basis to describe the thermodynamic cycles of many power plants, both from non-renewable sources, such as coal-fired thermoelectric plants, fuel oil or nuclear; and also, thermodynamic cycles with renewable sources, such as solar thermal power plants or geothermal power plants.

The image shows a thermal power station. In most of these plants, components such as regenerators are incorporated, whose purpose is to increase the efficiency of the cycle and improve its performance.
Basic components of the Rankine cycle
Although the Rankine cycle can incorporate various improvements and components, whose purpose is to increase the efficiency of the cycle; There are four basic devices that are required to complete the circuit. These are:
• The pump: it is the component in charge of increasing the pressure of the heat transfer fluid from the pressure minimum (operating pressure of the condenser), up to the maximum pressure (operating pressure of the boiler). The pumps can only work with substances in a liquid state and not with mixtures, and under ideal considerations, the process of Compression is performed isentropically, although in reality there is always an increase in entropy during compression. compression.
• The condenser: it is the system in charge of exchanging heat with a reservoir at low temperature (they can be rivers, lakes or other sources), in order to achieve a phase change of the steam (or mixture) at the turbine outlet, until it reaches a liquid state before entering the pump. Usually it is a coil or pipes through which the fluid circulates internally. work, and transfers heat to the fluid used as a cooling medium without actually mixing with this. Ideally, the condenser operates at constant pressure, although in practice, slight pressure drops occur during the condensation process. condensation.
• The boiler (or its equivalent): this is the element or space where the addition of heat to the system takes place, and this source of heat can come from various sources (burning of a fuel fossil, biomass burning, geothermal deposits, energy solar thermal, or the heat generated during nuclear fission). The high-pressure fluid must enter the boiler and this is in charge of supplying it with the necessary heat to bring it to a state of steam (or superheated steam) before being expanded in the turbine. Ideally, boilers operate at constant pressure, although in practice pressure drops occur during the heat addition process.
• The steam turbine: in thermodynamic cycles, the turbines fulfill the inverse function of the pumps, that is, their objective is to expand the steam at the boiler outlet to bring it to a pressure minor. During the expansion process, the impact of the steam particles on the turbine blades causes the rotor shaft to rotate producing mechanical energy, which, in turn, can be transformed into electric power when coupled with a generator. Under ideal conditions, the expansion process in the turbine is carried out isentropically, but due to irreversibilities, increases in enthalpy.
The elementary Rankine cycle
This cycle, in its elementary version, is made up of four processes: two isobaric and two isentropic, as shown in the figure. scheme. The area enclosed within the boundaries of the 4 states represents the net work of the cycle (wnet), which is directly related to the thermal efficiency of the cycle.
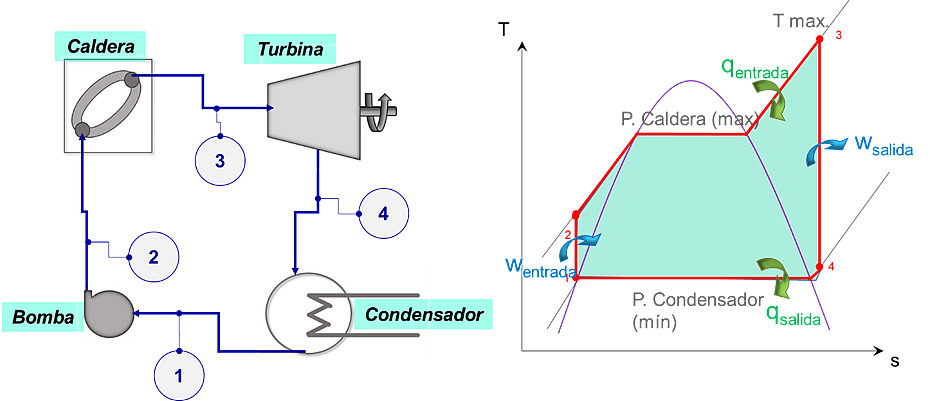
The ideal process followed by the working fluid (it can be water or another substance) is the following:
The substance in a liquid state enters the pump where it is compressed to the pressure of the boiler (state 2). In the boiler, the liquid is heated and changes phase, going from a liquid to a mixture and then to a vapor. If heat continues to be added beyond the saturated vapor state, the substance becomes a superheated vapor, increasing its temperature (state 3). Next, the steam enters the turbine to expand until it reaches the minimum pressure (state 4) and enter the condenser where it will lose heat to go from the state of vapor (or mixture) to liquid (state 4) completing the circuit.
Rankine cycle efficiency
The thermal efficiency is related to the area enclosed by the region delimited by the 4 states of the cycle, which which means that for constant heat input, the greater the net work, the greater the efficiency of the cycle. The net work (wnet) is the difference of the work generated by the turbine (wexit) minus the work done by the pump (wentrance). On the other hand, the efficiency of the cycle can also be increased by reducing the amount of heat that must be supplied to the boiler (qentrance), and one of the ways to achieve this is by incorporating heaters (open or closed) into the cycle, whose main function is to preheat the water from feeding (water that enters the boiler) through steam extractions from the turbine; this would make the circuit a regenerative Rankine cycle.

In the last equation, the variable h represents the enthalpy in each state, and the values are obtained from steam tables of the working fluid from pressure and/or temperature conditions.
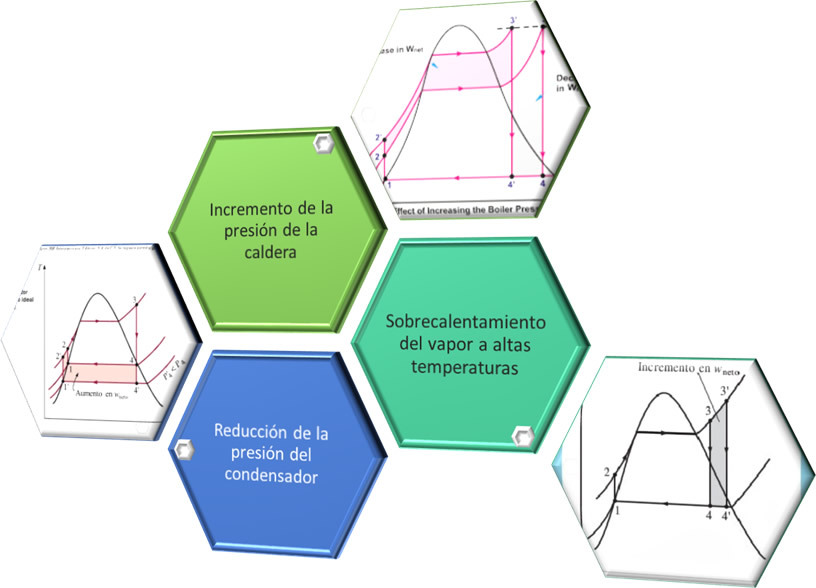
Improvements in the Rankine cycle are intended to increase the area that represents the net work of the cycle or to reduce the heat supplied by the boiler.

