50 Examples of Aldehydes and Ketones
Examples / / November 06, 2023
The aldehydes are organic compounds that have in their structure a carbonyl functional group (= C = O) that is linked to a carbon chain and a hydrogen atom. For example: methanal (also called formaldehyde), ethanal (also called acetaldehyde), and propanal (also called propaldehyde).
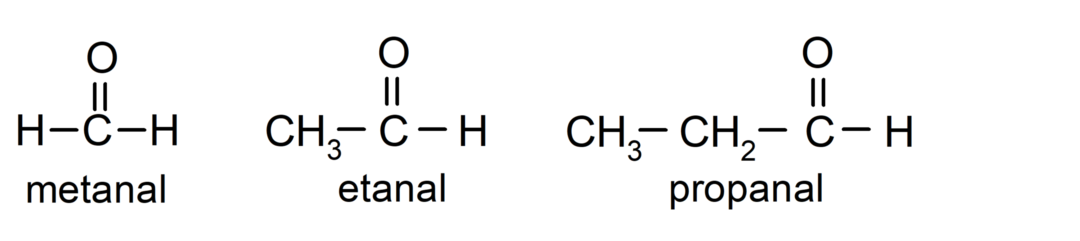
The ketones They are organic compounds that have in their structure a carbonyl group linked to two carbon atoms. For example: propanone (also called acetone), butanone and 2-pentanone.
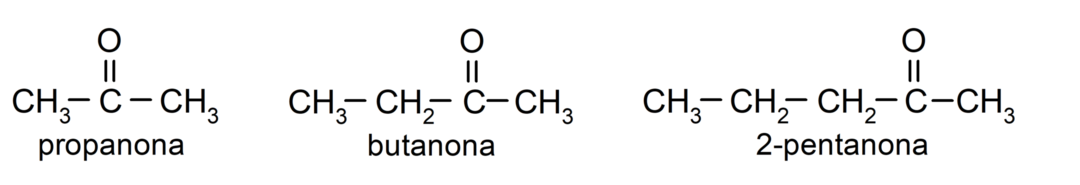
Differences between aldehydes and ketones
The main differences between aldehydes and ketones are:
- Aldehydes have in their structure a carbonyl functional group located at one terminal end, while ketones have the carbonyl group located in non-terminal positions of their structure.
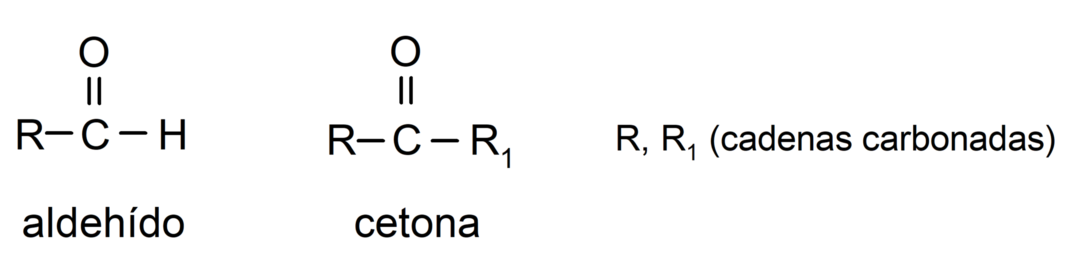
- Aldehydes are oxidized by reaction with Tollens' reagent, forming metallic silver. They also oxidize against Benedict's reagent, forming cuprous oxide. Ketones are not oxidized by either reagent.
- Aldehydes are reduced to primary alcohols, while ketones are reduced to secondary alcohols.
Physical properties
Physical properties of aldehydes
The physical properties of aldehydes are very diverse because they depend on the constitution of the carbon chain that is linked to the carbonyl group.
Some are:
- The aldehydes that are most soluble in water are those that are smaller in size, such as methanal and ethanal.
- Volatile aldehydes have pungent and even irritating odors.
- The carbonyl group gives them polarity.
- They usually have higher boiling points than chemical compounds of similar molecular size.
Physical properties of ketones
The physical properties of ketones depend on how the carbon chain that is linked to the carbonyl group is formed.
- Many ketones have pleasant odors.
- Its solubility in water depends on the size of the carbon chain attached to the carbonyl group. The smaller the carbon chain, the more soluble the ketone will be in water.
- The carbonyl group gives them a marked polarity.
- They have fairly high boiling points compared to chemical compounds of comparable molecular size.
Chemical properties
Chemical properties of aldehydes
Among the chemical properties of aldehydes we can find:
They are oxidized to form the corresponding carboxylic acid, that is, the acid formed will have the same number of carbons in the carbon chain as the aldehyde that gave rise to it. For example:
- Oxidation with Tollens reagent (ammoniacal silver complex in basic solution, [Ag (NH3)2]+) from ethanal produces ethanoic acid and metallic silver.
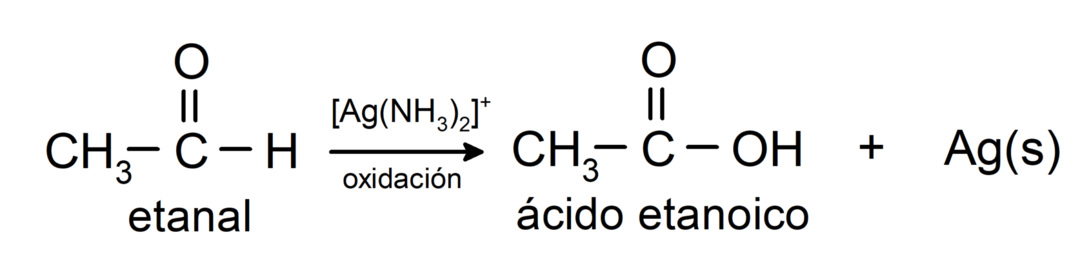
- Oxidation with Benedict's reagent (alkaline solution of copper sulfate) of ethanal produces ethanoic acid and cuprous oxide.
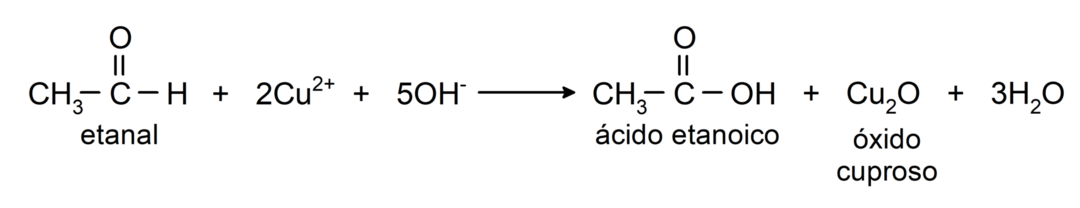
They undergo nucleophilic addition reactions, that is, addition of a nucleophile to the carbonyl group. For example:
- Addition of hydrocyanic acid to form cyanohydrins or cyanohydrins.
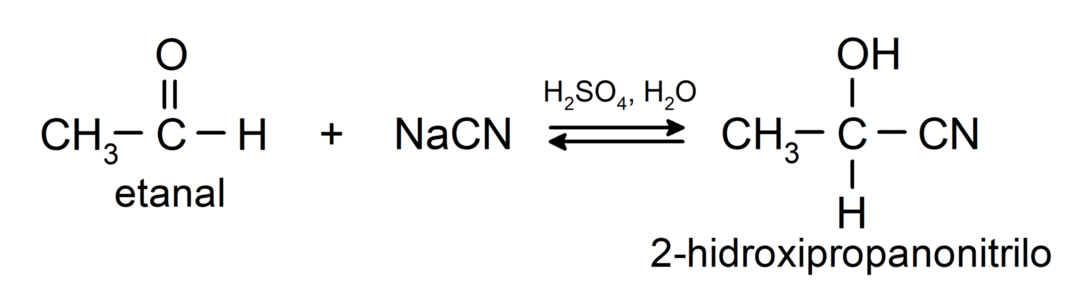
- In the presence of anhydrous acids, alcohols are added to the carbonyl group of aldehydes to form acetals and hemiacetals.
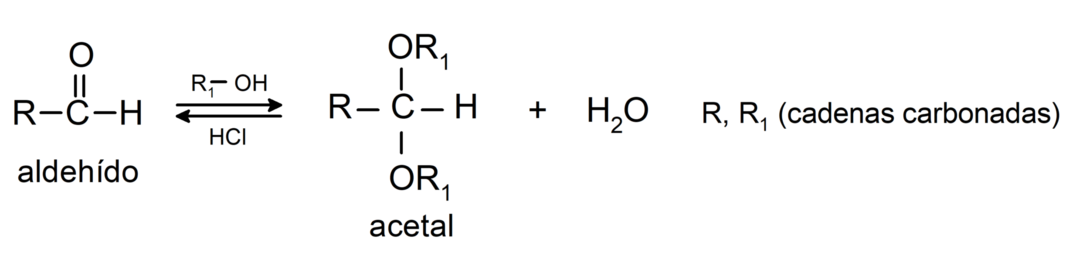
They undergo aldol condensation reactions. In these reactions, the union of two aldehydes occurs in the presence of sodium hydroxide (NaOH) and the chemical compound resulting is called aldol. For example:
- Condensation reaction of ethanal in the presence of diluted NaOH.
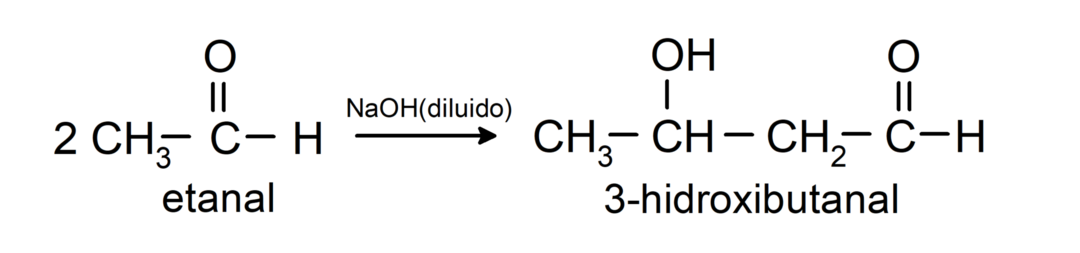
They are reduced to primary alcohols. Aldehydes can be reduced to primary alcohols by catalytic hydrogenation or by reduction with sodium borohydride (NaBH).4) and lithium aluminum hydride (LiAlH4).
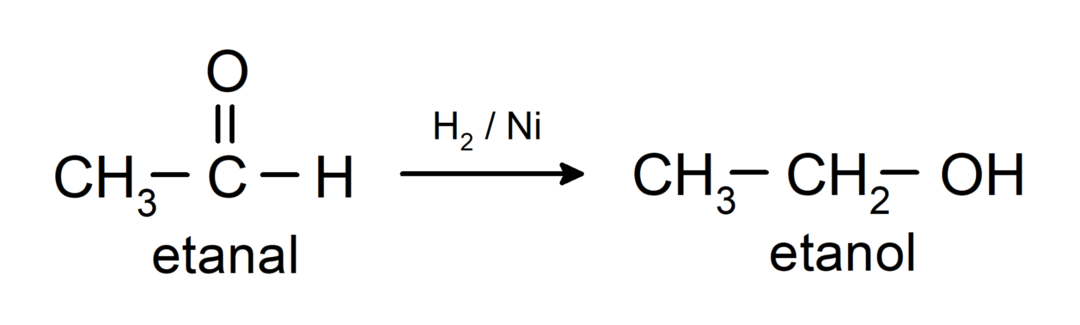
Chemical properties of ketones
Among the chemical properties of ketones we can find:
They undergo nucleophilic addition reactions. For example:
- Addition of hydrocyanic acid to form cyanohydrins or cyanohydrins.
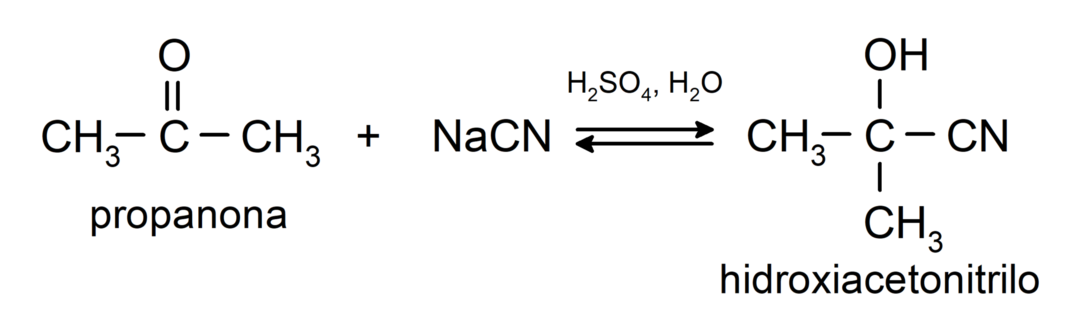
They add alcohols (in the presence of anhydrous acids) to the carbonyl group of ketones to form ketals and hemiketals. For example:
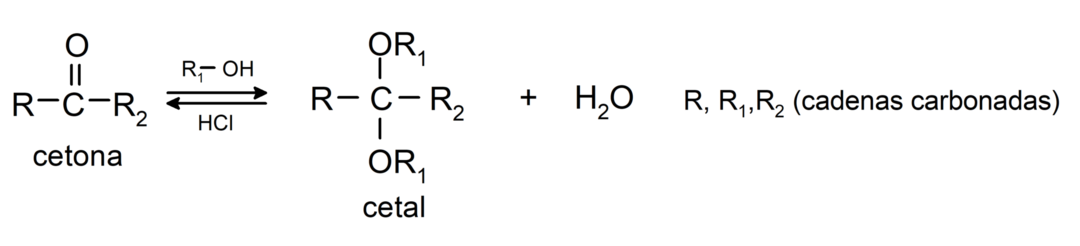
They undergo halogenation reactions. Ketones that have an alpha hydrogen (α) react by replacing this hydrogen with halogens (chlorine (Cl), bromine (Br), iodine (I), fluorine (F)) in the presence of acid or basic catalysts. Substitution occurs almost exclusively in carbon α, that is, the carbon that has bonded to hydrogen α. For example:
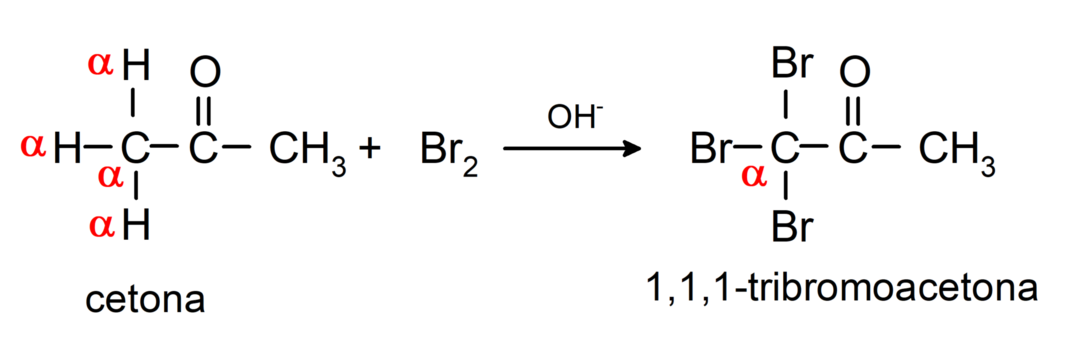
They are reduced to secondary alcohols by catalytic hydrogenation or by reduction with sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4). For example:
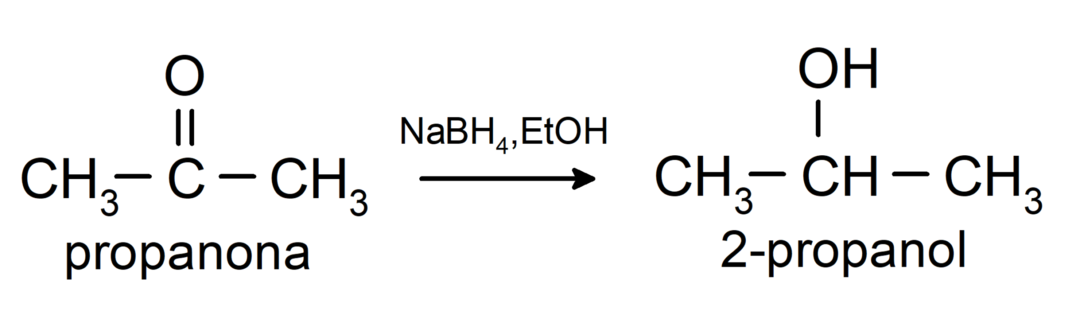
Ketones are not oxidized by Tollens and Benedict reagents.
Nomenclature of aldehydes
According to the rules established by the International Union of Pure and Applied Chemistry (IUPAC), Aldehydes are named using prefixes that indicate the number of carbons in the chain. carbonated. It is not necessary to specify the position of the carbonyl group, since it is always in position one, at one end of the molecule. Additionally, the suffix -al is written at the end of the aldehyde name. For example:
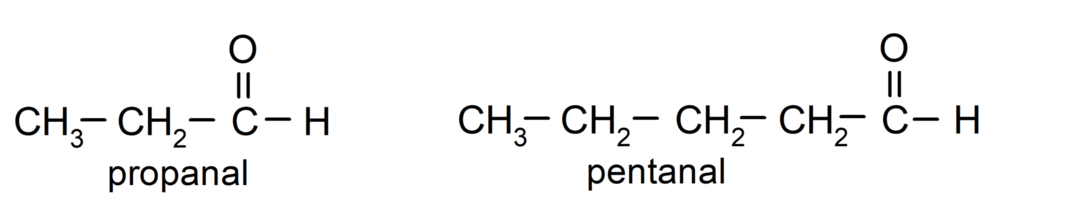
If the aldehyde is made up of several carbon chains, that is, it has branches, the carbon chain with the greatest number of carbon atoms is chosen as the main chain. The other chains are named as substituent groups, and the position of each substituent is chosen so that it occupies the smallest possible number in the chain. In addition, the carbon atoms begin to be counted starting from the end that has the carbonyl group. For example:
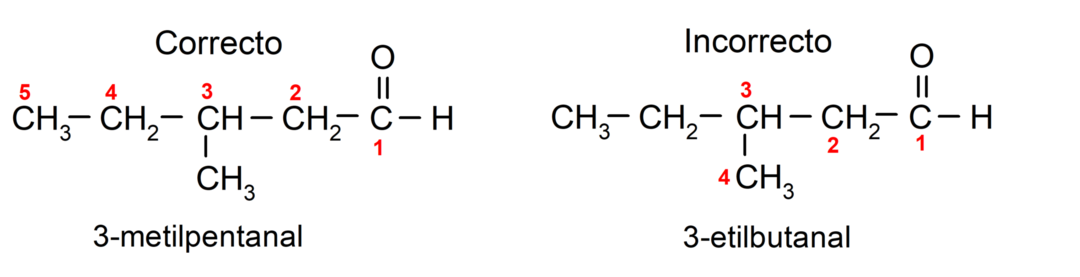
Aldehydes that have two carbonyl groups are named using the suffix -dial. For example:
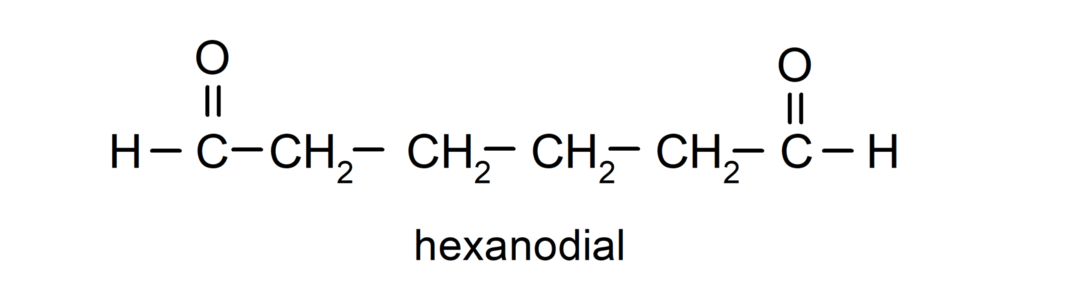
Ketone nomenclature
According to the International Union of Pure and Applied Chemistry (IUPAC), ketones are named using prefixes that indicate the number of carbons in the carbon chain.
On the other hand, the name of acetone is written using the suffix -one, preceded by a number that indicates the position of the carbonyl group in the carbon chain. The location of the carbonyl group should be chosen in such a way that it corresponds to the lowest numbering possible. For example:
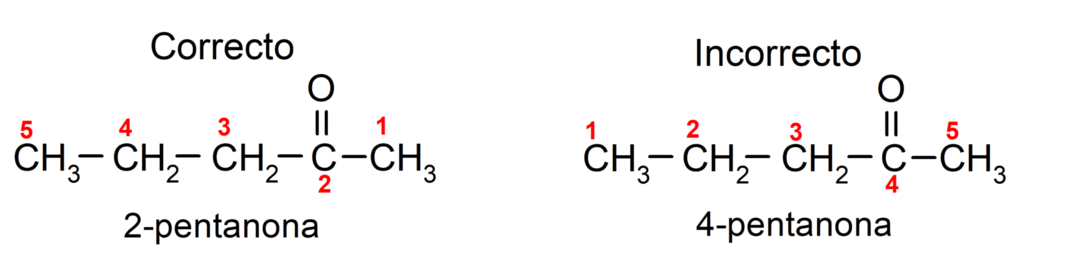
To name a ketone that is made up of several carbon chains, that is, with branches, we choose as the main chain, the carbon chain with the greatest number of carbon atoms and that contains the group carbonyl. The rest of the chains are named as substituent groups. For example:
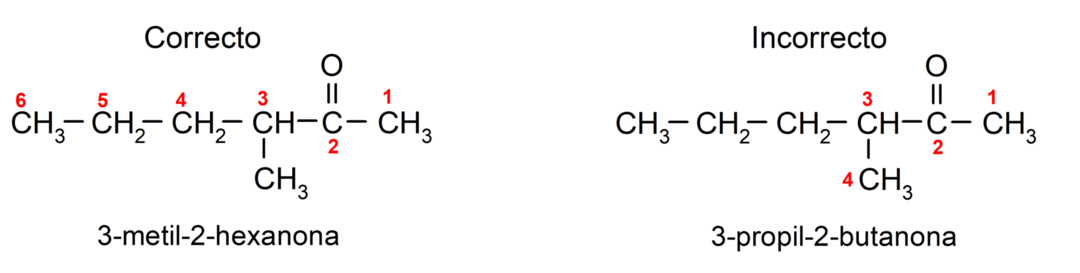
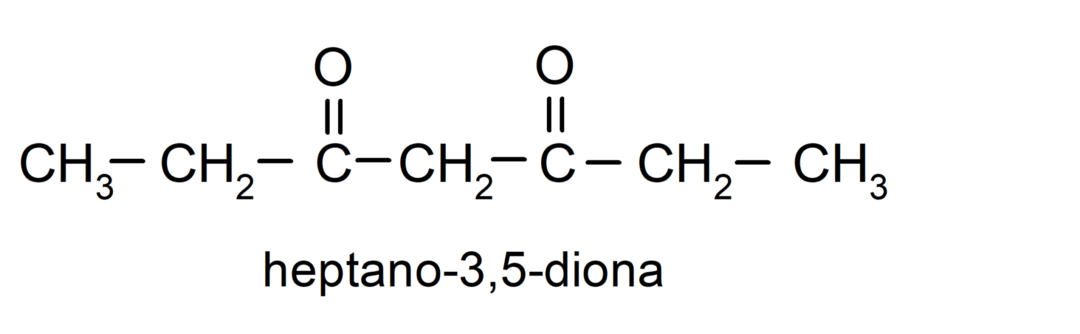
There are ketones that have two carbonyl groups, they are called diones. For example:
Examples of aldehydes
- methanal (formaldehyde)
- ethanal (acetaldehyde)
- propanal (propaldehyde)
- butanal
- pentanal
- hexanal
- 3-bromocyclopentanecarbaldehyde
- cyclohexanecarbaldehyde
- benzaldehyde
- 4,4-dimethylpentanal
- 2-hydroxy-butanal
- 2-hydroxy-2-methyl-butanal
- 2,3-dimethylpentanal
- pentanedial
- 4-hydroxy-3-methoxybenzaldehyde
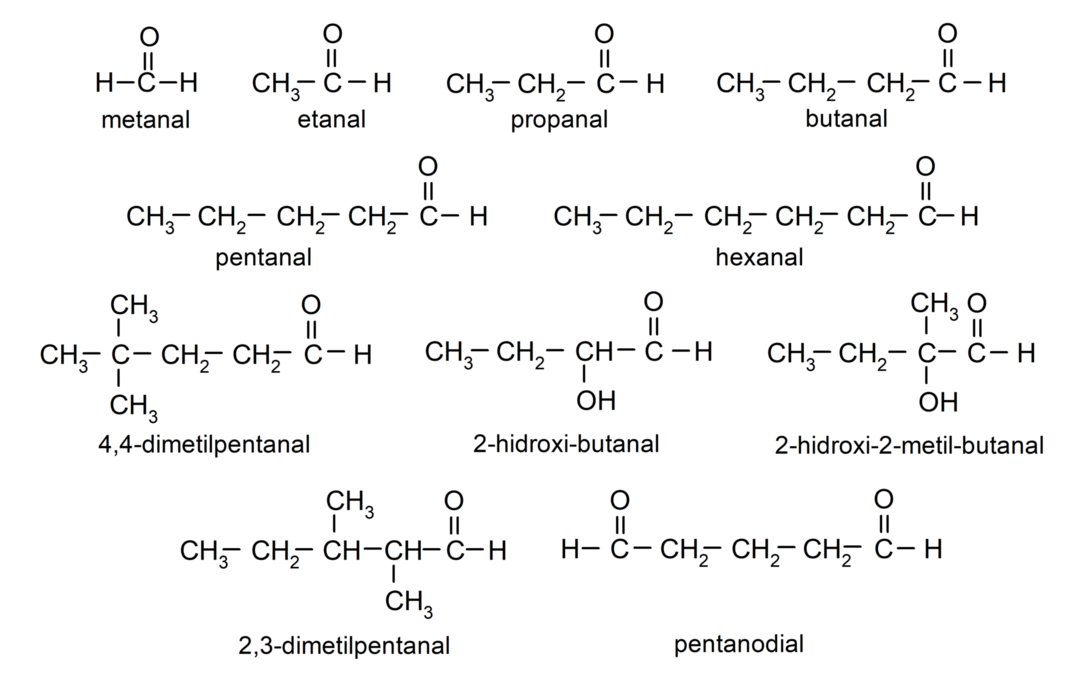
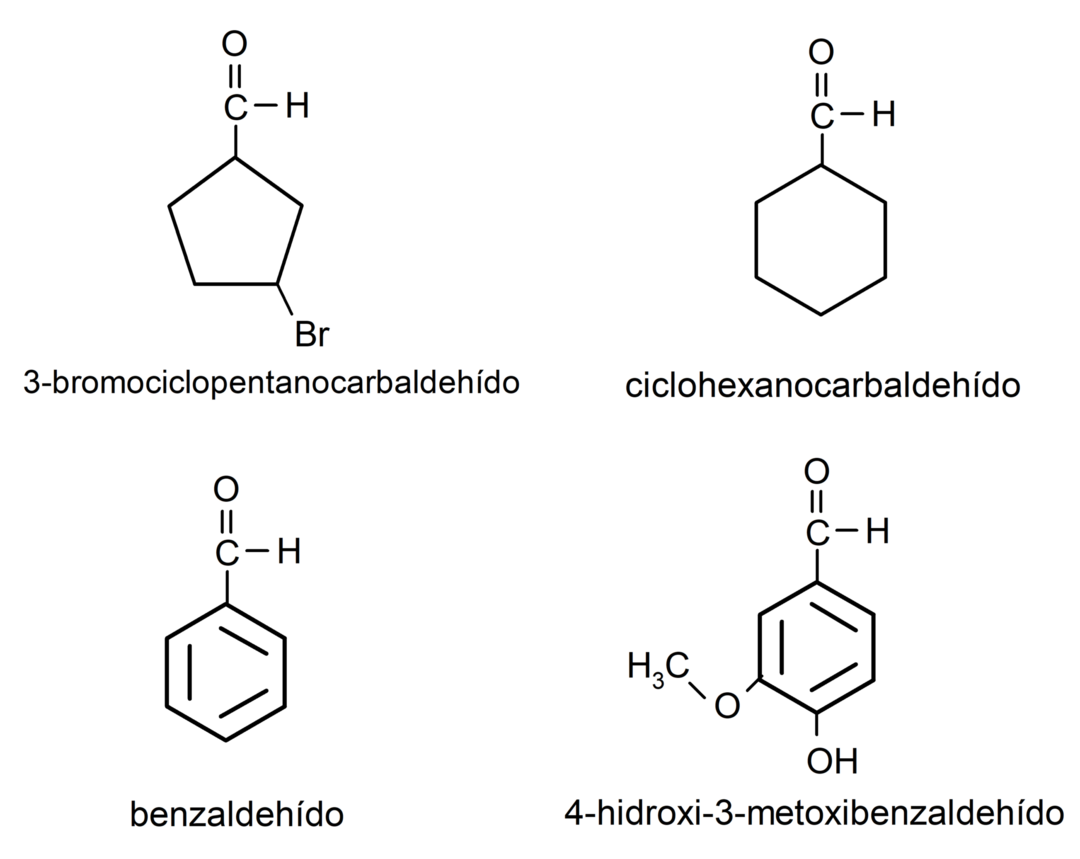
Examples of ketones
- propanone (acetone)
- butanone
- 2-pentanone
- 4-methylpentan-2-one
- 3-methylcyclohexanone
- cyclohexylmethylketone
- 3,4 dimethyl-hexan-2-one
- ethyl phenyl ketone
- 2,4-pentanedione
- cyclohexanone
- 3-pentanone
- 3-methyl-2,4-pentanedione
- 1-phenylpropanone
- cyclopentanone
- diphenyl ketone
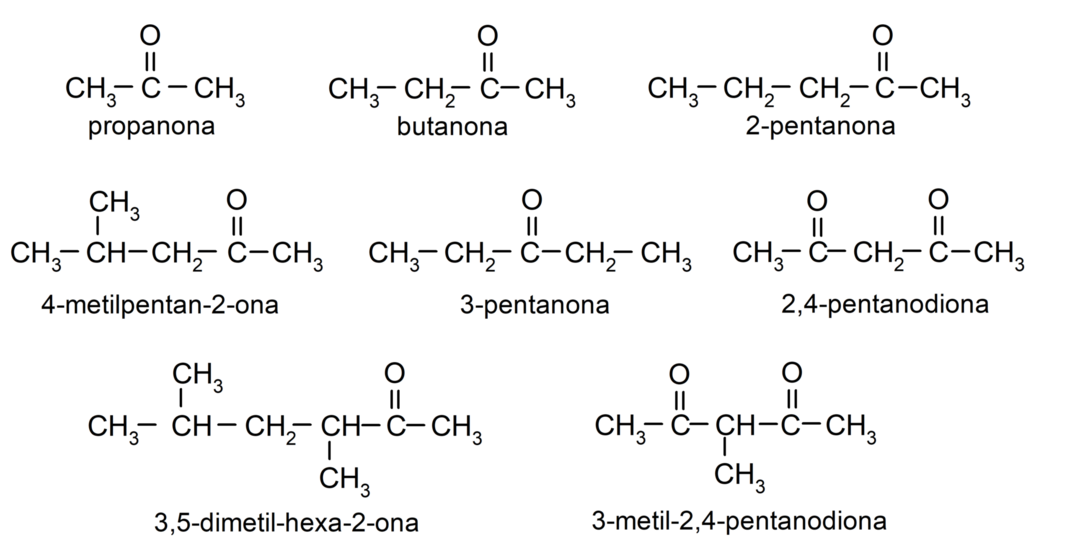
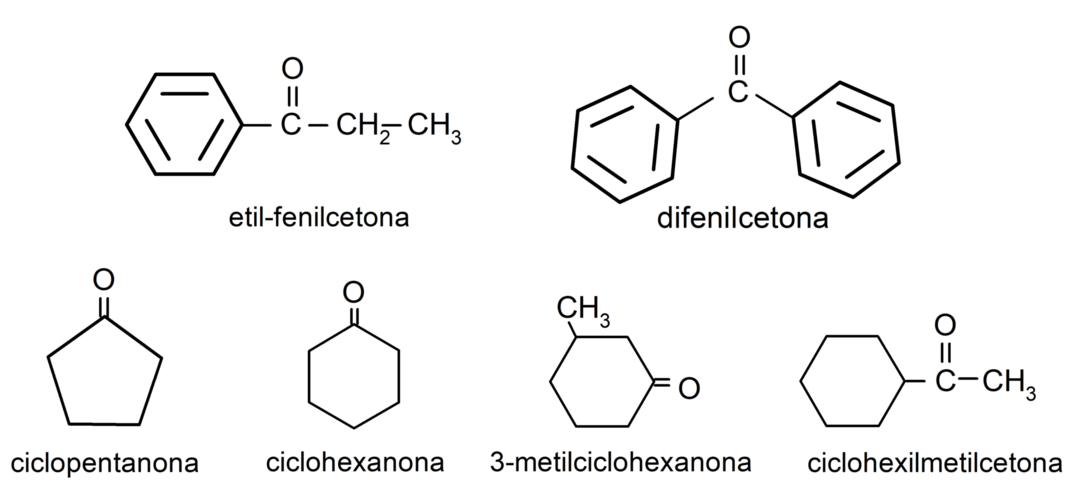
Uses of aldehydes in everyday life
Some uses of aldehydes are:
- They are used to produce solvents, paints, perfumes, resins and essences.
- They are used as preservatives in cosmetic products, biological samples and corpses. Formaldehyde is the most used for these purposes.
- They are used to produce plastics, which allow the replacement of metal parts in the automotive industry.
- They are used as flavorings for some foods.
- They are used as disinfectant agents.
- They have been used to make some explosives, such as pentaerythritol tetranitrate (TNPE).
Uses of ketones in daily life
Some uses of ketones are:
- They are used in the production of solvents. Especially acetone is widely used to remove paints and lacquers.
- They are used in the manufacture of some rubbers and lubricants.
- They are used to produce paints, lacquers and varnishes.
- They are used to produce medicines and cosmetics.
Aldehyde and ketone toxicity
- The aldehydes. Contact with aldehydes causes irritation to the skin, eyes and respiratory tract. Additionally, exposure to aldehydes has been linked to diseases such as cancer, contact dermatitis, and liver and neurodegenerative diseases. Formaldehyde, for example, is considered by the WHO (World Health Organization) to be a carcinogenic compound.
- ketones. Repeated exposure to ketones can cause damage to the central nervous system. This can lead to memory loss, weakness, muscle aches and cramps. Additionally, if the skin comes into contact with ketones, dryness and cracks occur. On the other hand, if ketones are inhaled, respiratory tract irritation and coughing occur.
References
- Llorens Molina, JA. (2018). “Aldehydes and ketones: Some examples.” http://hdl.handle.net/
- Gabriel Pinto Cañón, Manuela Martín Sánchez, José María Hernández Hernández, María Teresa Martín Sánchez (2015) “The Tollens reagent: from the identification of aldehydes to their use in nanotechnology. Historical aspects and didactic applications.”Vol. 111 No. 3. Royal Spanish Society of Chemistry.
- William Bauer, Jr. (2000) «Methacrylic Acid and Derivatives» in Ullmann’s Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. DOI: 10.1002/14356007.a16_441.
- “They reveal a new mechanism of toxicity in a group of carcinogenic compounds derived from diet and the environment” (2022) In: www.conicet.gov.ar Available in: https://www.conicet.gov.ar/ Accessed: June 20, 2023.
Follow with:
- Alcohols
- Sugars
- Alkanes

