20 Examples of Amides
Examples / / November 06, 2023
The amides are organic chemical compounds derivatives of carboxylic acids, where the hydroxyl group (-OH) of the carboxyl group (-COOH) of the acid is replaced by an amino group (-NH2, -NH-R, -N-(R)2, R being any carbon chain).
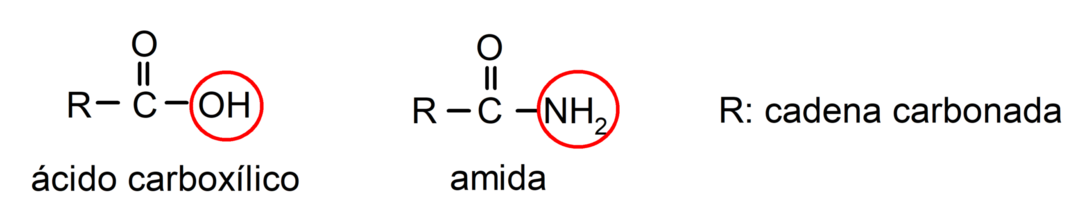
Amides are organic molecules that contain the amide group, which is made up of a carbonyl group and an amino group. For example: etanamide, propanamide and N-methyl-ethanamide.

- See also: Aldehydes and ketones
Types of amides
Amides can be classified according to the number of hydrogens (which are attached to the nitrogen of the amino group) that have been replaced by different substituent groups. In this sense, there are primary, secondary and tertiary amides.
- Primary amides. They are amides that do not have the hydrogens of the amino group substituted. For example:

- Secondary amides. They are amides that have one of the hydrogens of the amino group substituted. For example:
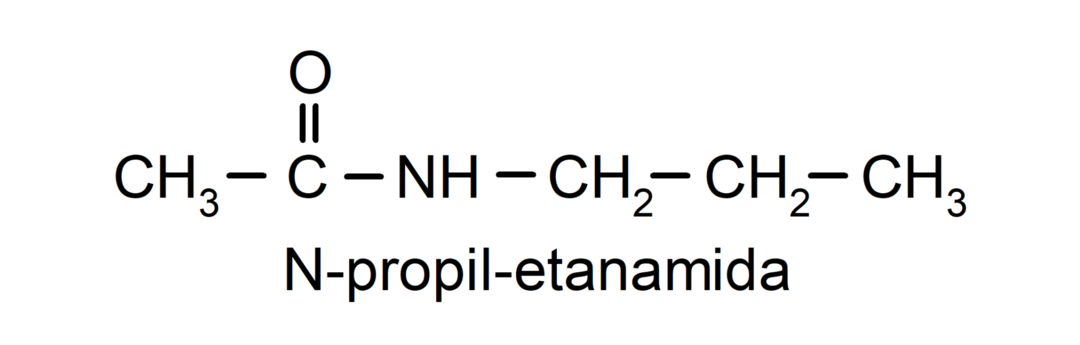
- Tertiary amides. They are amides that have two of the hydrogens of the amino group substituted. For example:

Nomenclature of amides
According to common nomenclature, amides are named as derivatives of carboxylic acids following the following rules:
- The carbon corresponding to the carbonyl group is located in position 1 and from this carbon the longest carbon chain is chosen. For example:

- Primary amides are named using the prefix corresponding to the number of carbon atoms of the carboxylic acid that gave rise to them. For example:

According to the International Union of Pure and Applied Chemistry (IUPAC), amines They are named using the following rules:
- Secondary and tertiary amides are named using the prefix corresponding to the number of carbon atoms of the carboxylic acid that gave rise to them. Furthermore, for each hydrogen of the amino group that has been substituted, an N is placed. Thus, the different substituents are named indicating their quantity and at the end of the name the word amide is placed. For example:

- When the molecule has priority groups with respect to the amide group, then the amide is named as a substituent. In these cases the amide group is named carbamoyl. For example:
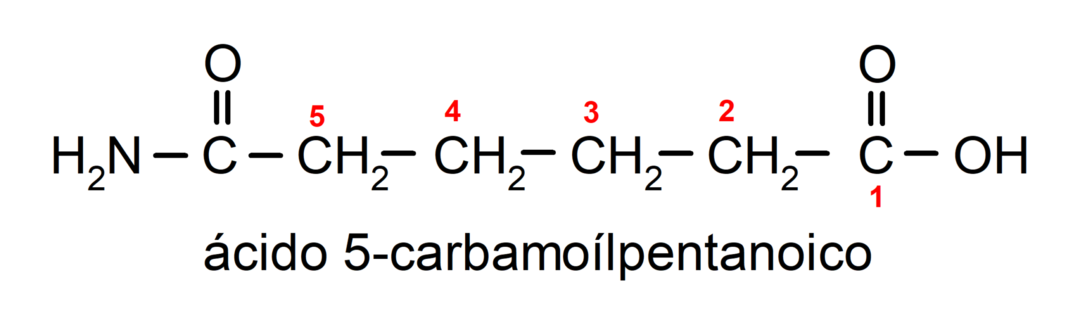
- When the molecule is formed by a cycle and an amide group, the cycle is taken as the main chain and the suffix -carboxamide is placed. For example:

Physical properties of amides
- Amides are solid at room temperature, with the exception of methanamide.
- They have high boiling points, even higher than those of the corresponding carboxylic acids.
- Amides are good solvents.
- They are weak bases.
Chemical reactions of amides
- Amides react with an aqueous acid to form the corresponding carboxylic acid and an ammonium salt. For example:

- Amides react with alkali to form the corresponding carboxylic acid and a carboxylate salt. For example:
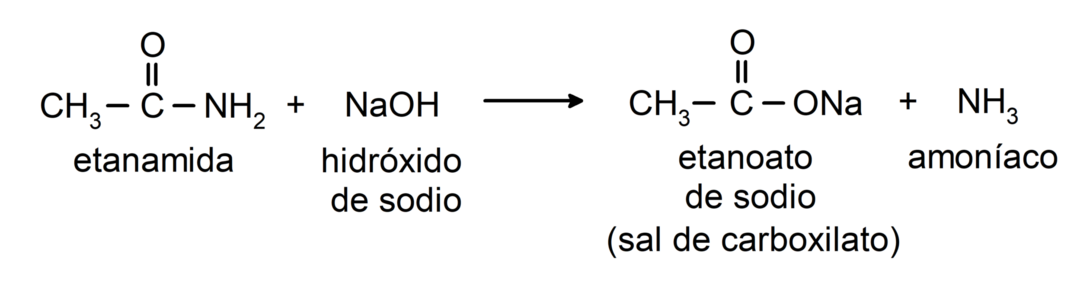
- Amides are reduced to amines in the presence of lithium aluminum tetrahydride:
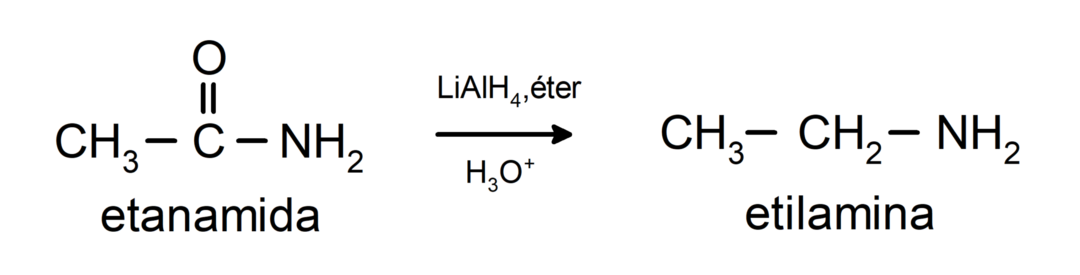
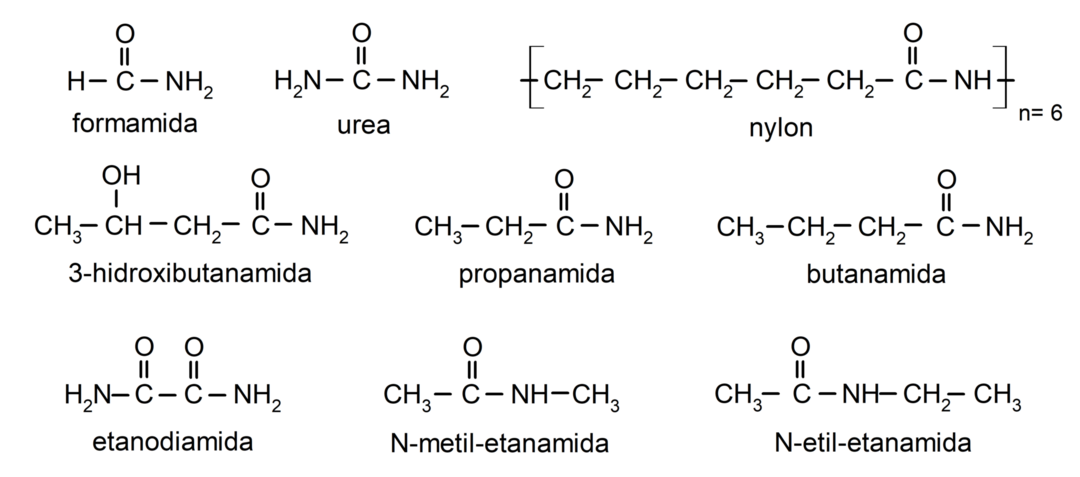
Examples of amides
- formamide
- urea
- nylon
- ε-caprolactam
- etanamide
- propanamide
- butanamide
- ethanediamide
- N-methyl-ethanamide
- N-ethyl-ethanamide
- N-propyl-ethanamide
- N, N-dimethyl-butanamide
- benzenecarboxamide
- 4-bromo-3-methyl-cyclohexanecarboxamide
- 3-hydroxybutanamide

Uses of amides
Amides are widely used in the pharmaceutical industry. In addition, they are used as mold release components in the plastics industry. On the other hand, they are used as emulsifiers, surfactants and solvents. For example, urea is an amide that is widely used in the pharmaceutical industry and the nylon industry.
References
- Ramírez-Barrón, S. N., Sáenz-Galindo, A., López-López, L., & Cantú-Sifuentes, L. (2013). Amides, Application and Synthesis. Scientific Magazine of the Autonomous University of Coahuila, 5(9).
- Caglieri, S. C., & Pagnan, M. (2013). Theoretical study on the acid hydrolysis of aliphatic and aromatic amides. Technological information, 24(3), 35-40.
- Martinez, C. H. M., Gomez, L. AND. P., de Escobar, M. S., & Escalante, F. TO. (2002). organic chemistry. University of Las Palmas de Gran Canaria, Vice-Rectorate of Studies and Teaching Quality.
Follow with:
- Aldehydes
- Alcohols
- Macronutrients and micronutrients
- Organic and inorganic compounds

