20 Examples of Amines
Examples / / November 06, 2023
The amines are organic chemical compounds ammonia derivatives (NH3), where at least one of the hydrogens of ammonia has been replaced by an alkyl or aryl group, resulting in the formation of an amino group (-NH2, -NH-, -N=). For example:methylamine, ethylamine and propylamine.

An alkyl group is a substituent that forms when a hydrogen is removed from a saturated hydrocarbon. In this way, the carbon from which that hydrogen was separated can bond to another atom. For example: methyl (CH3 -) and ethyl (CH3 – CH2 -).
An aryl group is a substituent derived from an aromatic ring, such as benzene. For example: phenyl (C6h5 -)
- See also: Amides
Types of amines
Amines can be classified according to the number of hydrogen atoms bonded to the nitrogen that have been replaced by alkyl or aryl functional groups.
According to this criterion, there are:
- primary amines. They are formed when only one of the hydrogen atoms of ammonia is replaced by an alkyl or aryl group. For example: ethylamine and 1-pentylamine.
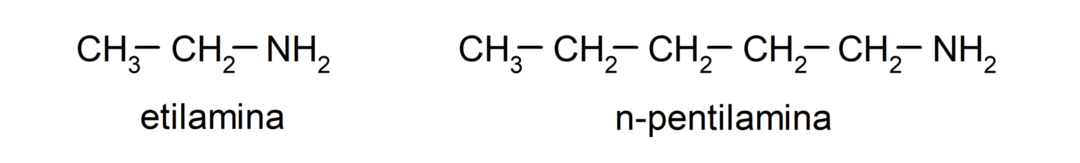
- secondary amines. They are formed when two of the hydrogen atoms of ammonia are replaced by an alkyl or aryl group. For example: diethylamine and ethylmethylamine.
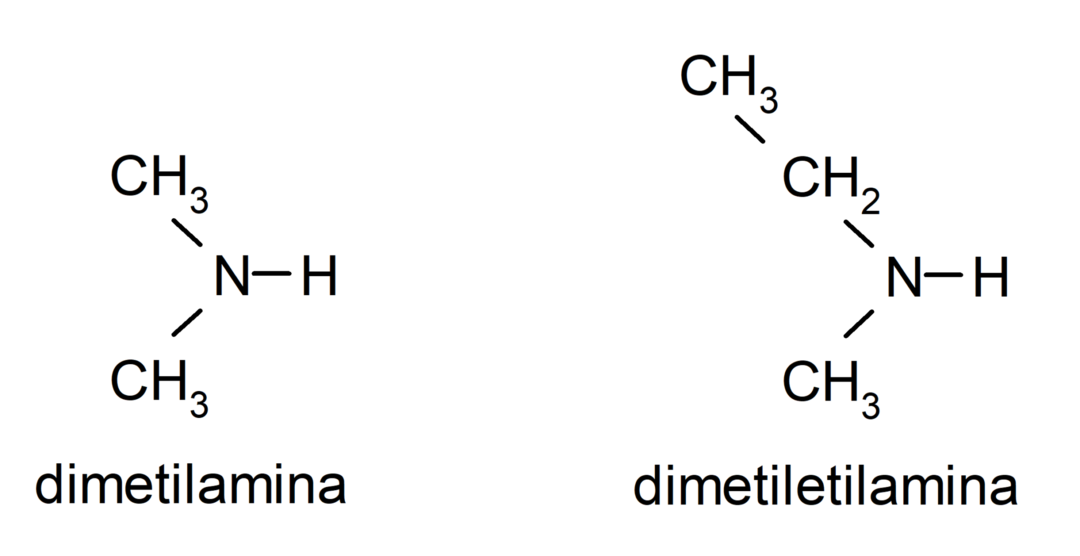
- Tertiary amines. They are formed when the three hydrogen atoms of ammonia are replaced by an alkyl or aryl group. For example: trimethylamine and dimethylethylamine.
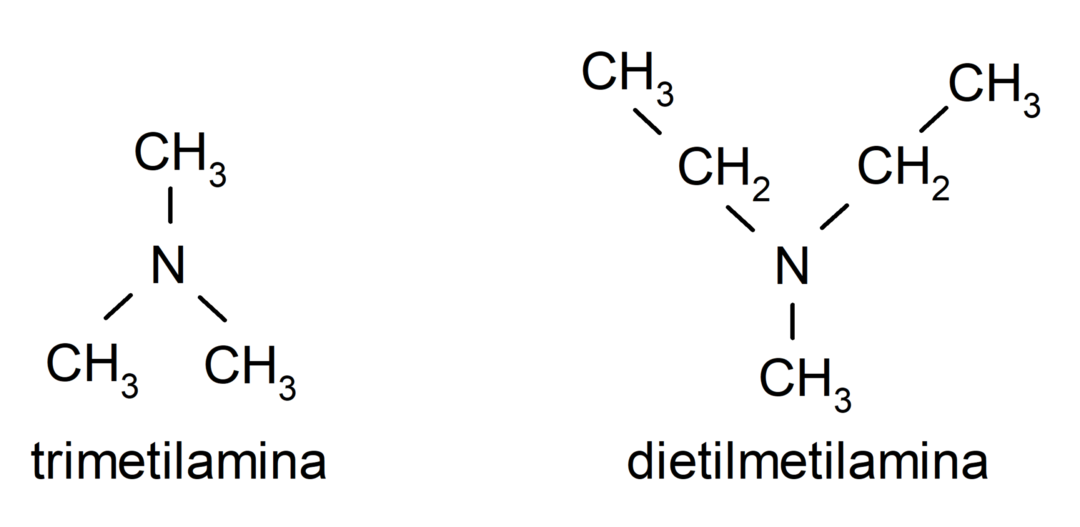
Amines are also classified based on the type of group that is attached to the nitrogen. According to this criterion, there are:
- Aliphatic amines. They have alkyl substituents. For example: butylamine and diethylamine.

- aromatic amines. They have aryl substituents. For example: phenylamine (aniline) and diphenylamine.
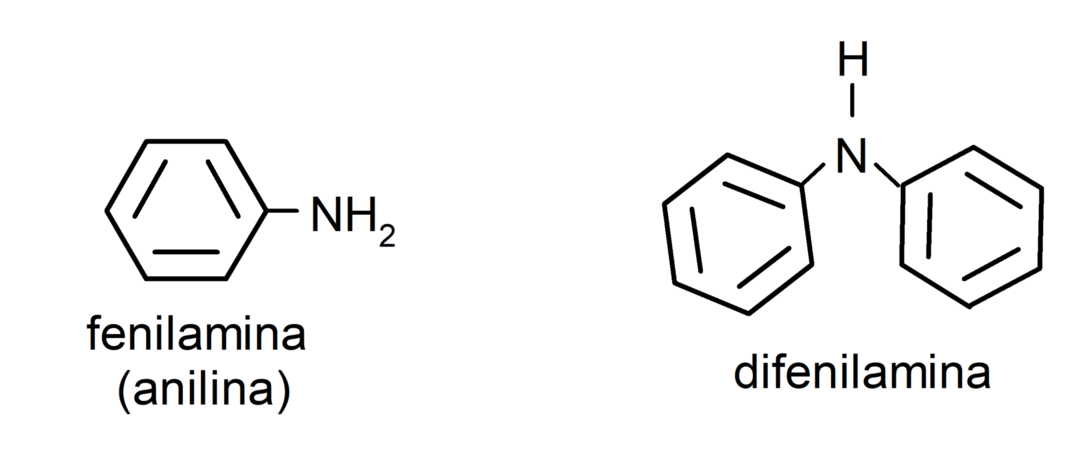
Nomenclature of amines
According to IUPAC (International Union of Pure and Applied Chemistry), amines are named following the following rules:
For the simplest amines, each of the substituents of the hydrogen groups bonded to the ammonia nitrogen is named. Then the word “amine” is placed at the end of the name.
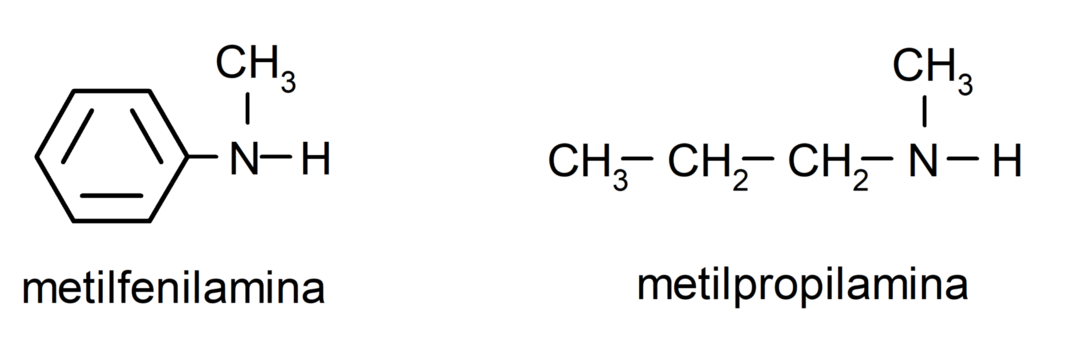
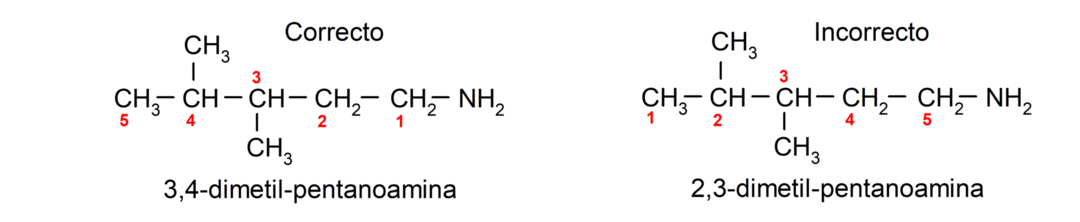
For amines that contain several carbon chains (which, in turn, have other substituents), the carbon chain that contains the amino group is chosen as the main one. Furthermore, the position of the amino group is chosen so that it occupies the smallest position in the carbon chain, and in Based on this position, the substituents are also chosen so that they occupy the smallest possible position in the chain.
Finally, they are named using the name of the corresponding alkane, alkene or alkyne for the main chain, and the amine suffix is placed.
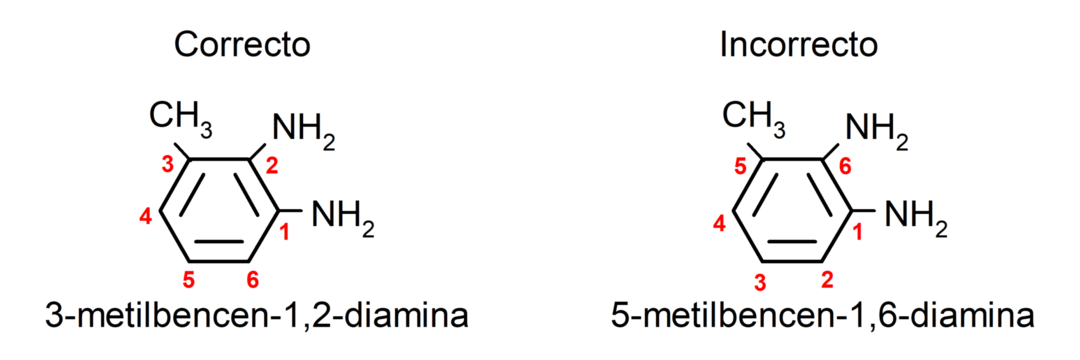
If the carbon chain contains several amino groups, they are chosen in the position they occupy in the smallest combination of the numbering of their positions. In addition, the name of the corresponding alkane, alkene or alkyne is placed, followed by the term that indicates the number of amino groups, followed by the suffix “amine”.
Physical properties of amines
The physical properties of amines depend largely on how many hydrogens attached to the nitrogen have been substituted, and by what type of substituents they have been substituted.
- Solubility. In general, amines are more soluble in water than the corresponding saturated hydrocarbons. Furthermore, amines with long carbon chains are less soluble in water than amines with short carbon chains. On the other hand, aromatic amines are not soluble in water.
- Boiling point. The boiling point of amines is higher than that of alkanes with the same number of carbon atoms.
- Smell. Most amines have unpleasant odors. For example, putrescine smells like rotting meat and trimethylamine smells like rotting fish.
Chemical properties of amines
Some chemical properties of amines are:
- They are basic chemical compounds, that is, they undergo reactions where they accept protons according to the Brønsted-Lowry Theory. For example:
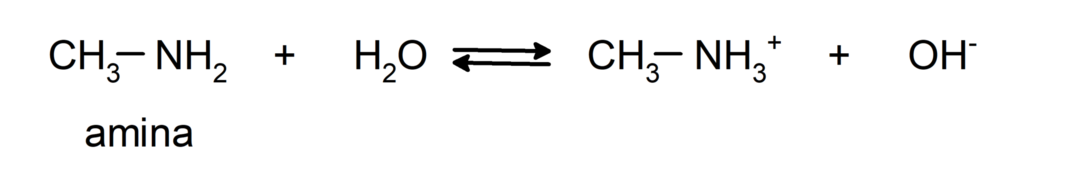
- They present neutralization reactions with acids in which amine salts are formed. For example:

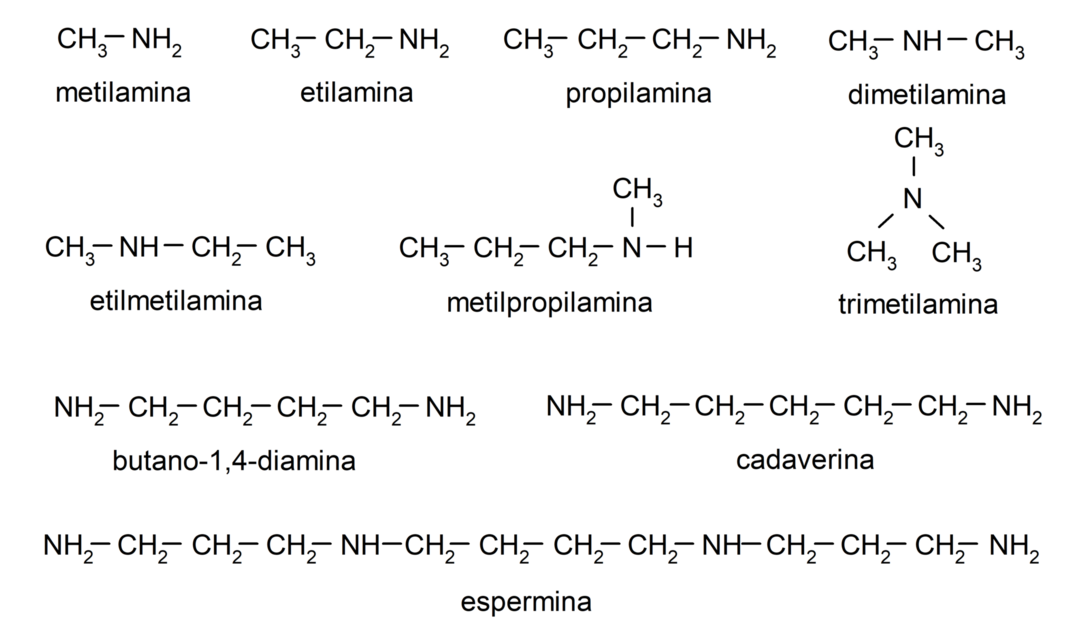
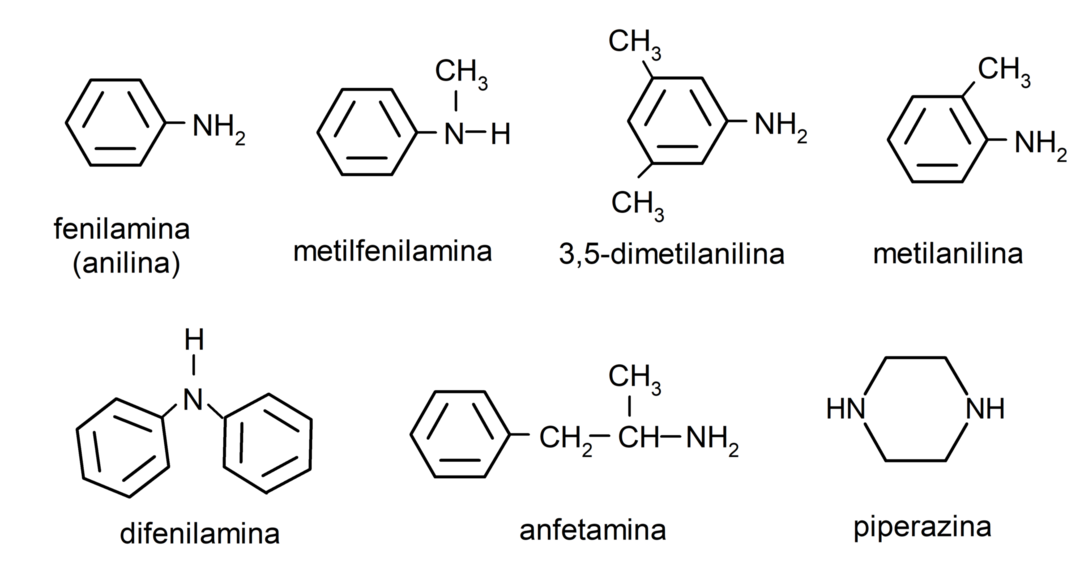
Examples of amines
- methylamine
- ethylamine
- propylamine
- dimethylamine
- ethylmethylamine
- phenylamine (aniline)
- diphenylamine
- methylpropylamine
- butane-1,4-diamine
- spermine
- cadaverine
- 3,5-dimethylaniline
- methylaniline
- amphetamine
- piperazine
Biological function of amines
Amines are present in many living beings and perform various functions. Some amines, such as epinephrine, norepinephrine, serotonin, and dopamine, are neurotransmitters.
The amino group is one of the functional groups that form amino acids, which are the fundamental units of proteins. Additionally, the nitrogenous bases that make up DNA and RNA contain amino groups.
Uses of amines
Some uses of amines are:
- They are used to produce agrochemical and pharmaceutical compounds.
- They are used as catalysts in the production of polyurethanes.
- Due to their damping effect, they are used to inhibit corrosion in aqueous environments.
- They are used to purify gases in power plants and refineries.
- They are used to make cleaning products.
- They are used to produce personal care products, as they improve foaming in soaps and shampoo.
Amines Toxicity
Inhalation of amines in high concentrations causes poisoning, which in turn causes increased blood pressure and seizures. Furthermore, contact with aliphatic amines in gaseous state causes irritation to the eyes and respiratory tract. Several amines cause skin burns upon contact.
Aromatic amines are very toxic, but since they are much less volatile than aliphatic amines, contact with them can be better controlled.
References
- Pérez, C. R. C., & Jiménez-Colmenero, F. (2010). Biogenic amines: Toxicological importance. Electron. J. Biomed., 3, 58-60.
- Fernández García, M., & Álvarez González, M. Á. (2005). Biogenic amines in foods.
Follow with:
- Alcohols
- Aldehydes
- Ethyl alcohol
- organic chemistry

