30 Examples of Alcohols
Examples / / November 06, 2023
The alcohols are organic chemical compounds that contain in their structure the hydroxyl functional group (- OH) attached to a carbon (- C). The group (- C – OH) is called “carbinol”. Some examples of alcohols are: methanol, ethanol and 1-propanol.
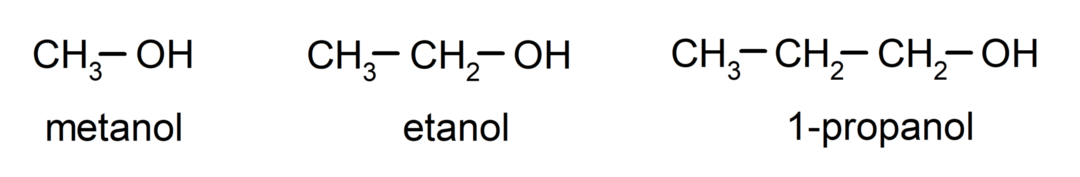
Alcohols are chemical compounds with various uses in everyday life, as they have antibacterial and antiseptic properties. On the other hand, they can be dangerous to human health when ingested uncontrolled.
Likewise, not all alcohols can be ingested by humans.
- See also: Amines and ketones
Types of alcohols
Depending on the number of carbon atoms to which the carbon atom that has the hydroxyl group attached is attached, an alcohol can be:
- Primary alcohol. The carbon atom that has the hydroxyl group attached is also attached to a single carbon atom. For example:
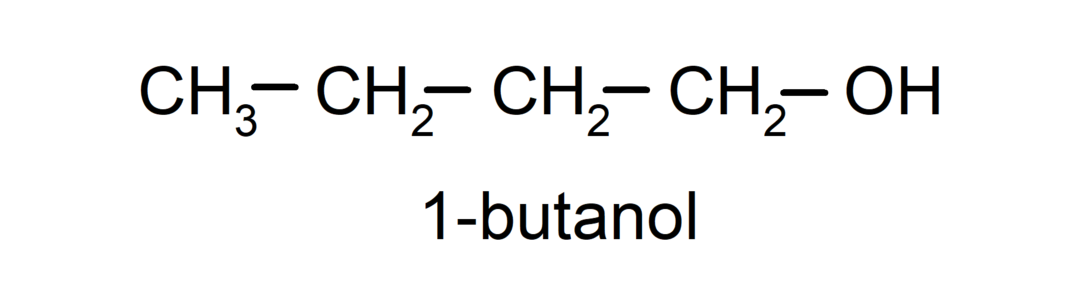
- Secondary alcohol. The carbon atom that has the hydroxyl group attached is also bonded to two other carbon atoms. For example:
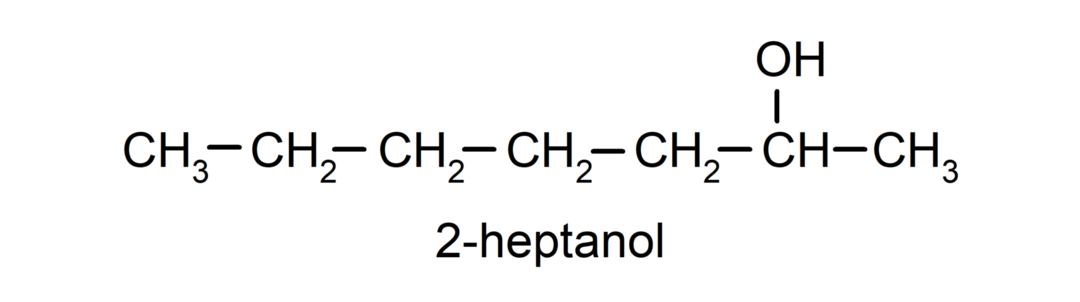
- Tertiary alcohol. The carbon atom that has the hydroxyl group attached is also attached to three carbon atoms. For example:
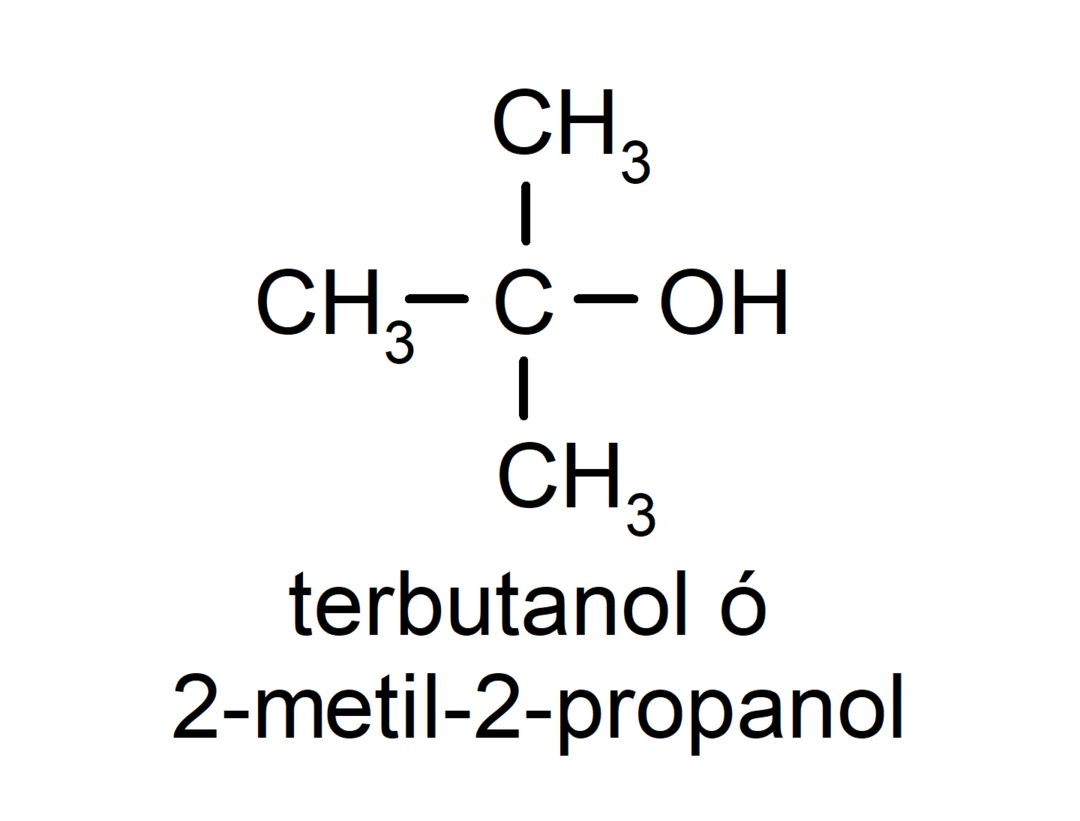
Depending on the number of hydroxyl groups it has, an alcohol can be:
- Diol. It has two hydroxyl groups in its structure. For example:
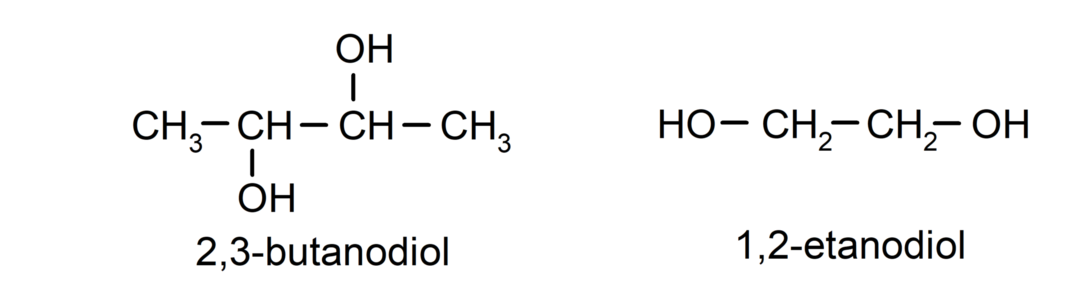
- Triol. It has three hydroxyl groups in its structure. For example:
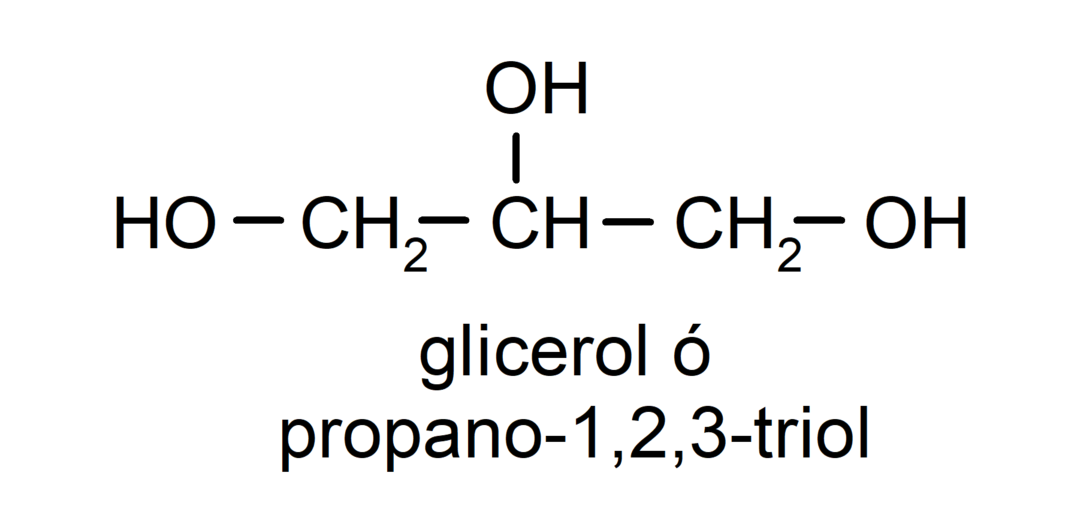
- Polyalcohol. It has many hydroxyl groups in its structure. For example:
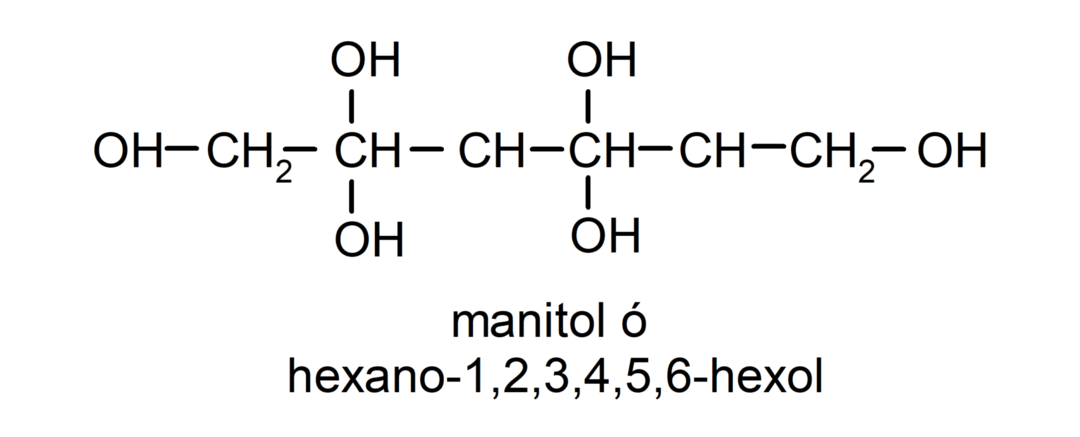
Physical properties of alcohols
Some of the physical properties of alcohols are:
- Boiling point. The boiling point in alcohols is quite high due to the presence of the hydroxyl group, which allows the formation of hydrogen bonds. Furthermore, the more hydroxyl functional groups are present in the carbon chain, the higher the Boiling point of alcohols.
- Polarity. Alcohols are quite polar compounds.
- Solubility. Low molecular weight alcohols are soluble in water. On the other hand, the larger the carbon chain of alcohols, the lower their solubility in water. Additionally, the more hydroxyl groups alcohols have, the greater their solubility in water.
- State of aggregation. Most alcohols are liquid at room temperature (25ºC) and have characteristic odors.
Chemical properties of alcohols
Some of the chemical properties of alcohols are:
- Alcohols behave like acids and bases.. Their behavior as acids can be seen in reactions with active metals to release hydrogen gas and form alkoxides.

Their behavior as bases can be seen in reactions such as the reaction of methanol with hydrogen bromide to form methyloxonium bromide.
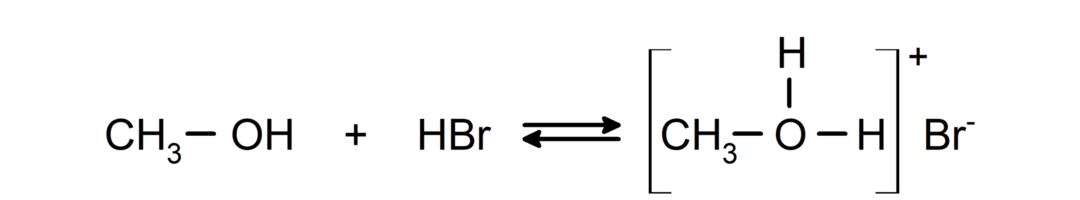
- Alcohols undergo halogenation reactions. They react with hydrogen halides to form alkyl halides.
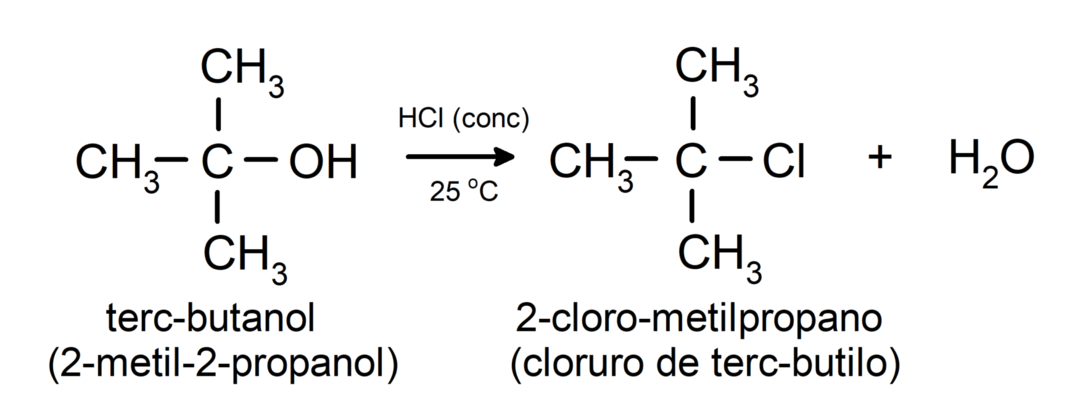
-
Alcohols undergo oxidation reactions when they react with certain oxidizing compounds. The products of oxidation reactions depend on the type of alcohol reacting, that is, whether it is primary, secondary or tertiary.
Oxidation of primary alcohol to form aldehyde or carboxylic acid.
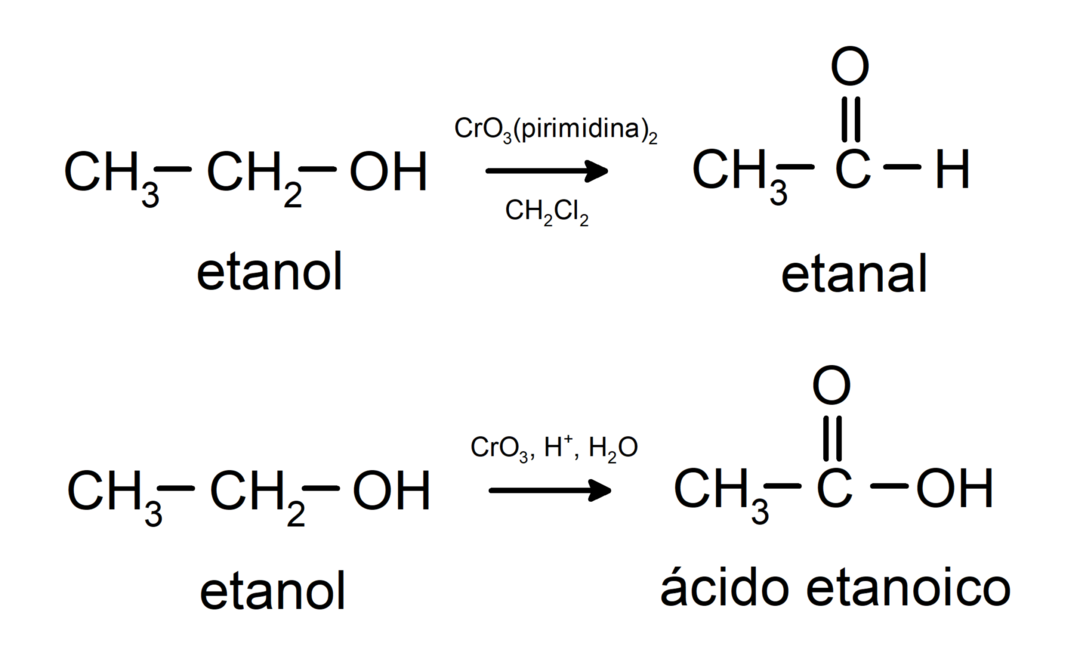
Oxidation of secondary alcohol to form ketone.
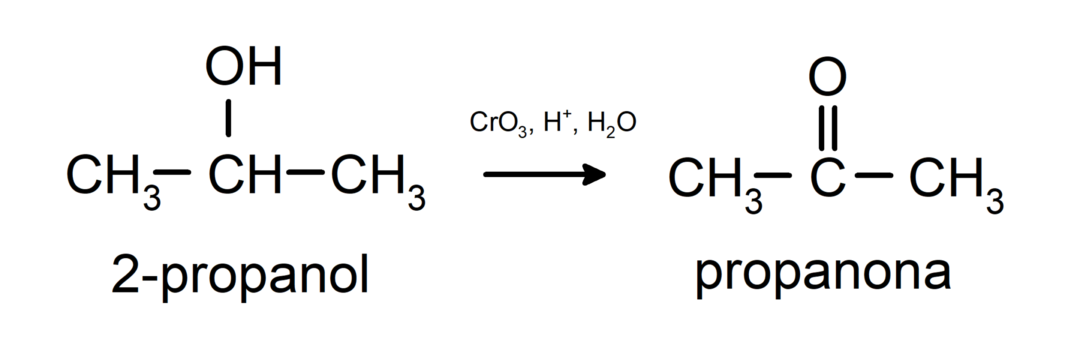
Tertiary alcohols do not oxidize with common oxidants. With very strong oxidizing agents they can be transformed into alkenes, which can then be oxidized.
Alcohols that have two hydroxyl groups located on adjacent carbons are oxidized with lead tetraacetate to form two ketones.
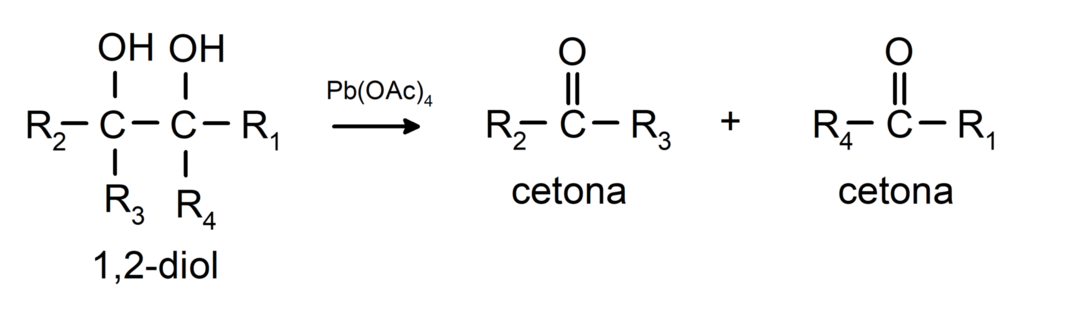
- Alcohols undergo dehydrogenation reactions. These reactions occur only with primary and secondary alcohols, which when subjected to high temperatures in the presence of catalysts, release hydrogens.
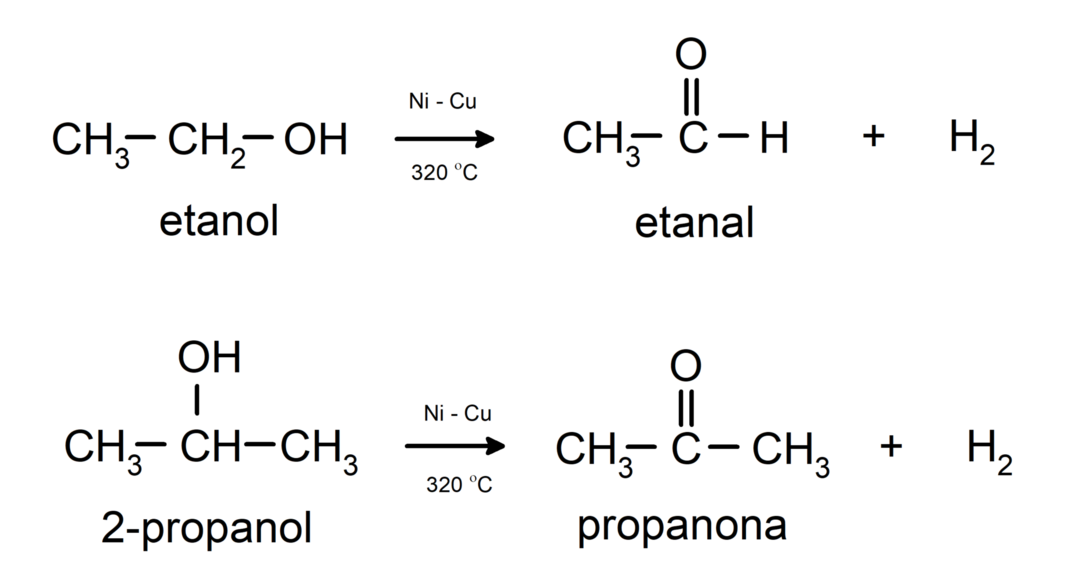
- Alcohols undergo dehydration reactions. Alcohols dehydrate to form the corresponding alkenes. This reaction occurs in the presence of acid and intermediate temperatures.
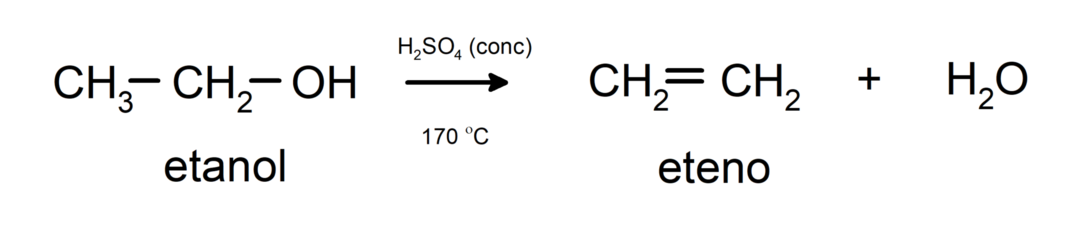
Alcohol nomenclature
According to the nomenclature established by the IUPAC (International Union of Pure and Applied Chemistry), alcohols are named following the following rules:
- The position of the hydroxyl group is chosen taking into account that it occupies the lowest possible number in the carbon chain. If the structure of the alcohol is cyclic, the 1 position on the carbon that has the hydroxyl group attached is considered and the prefix cyclo- is used to name it.
- The name of the alcohol is written using prefixes that indicate the number of atoms in the carbon chain, and in addition, the suffix -ol is placed.
- If the structure of the alcohol has branches, the longest chain that also contains the hydroxyl group is chosen as the main carbon chain.
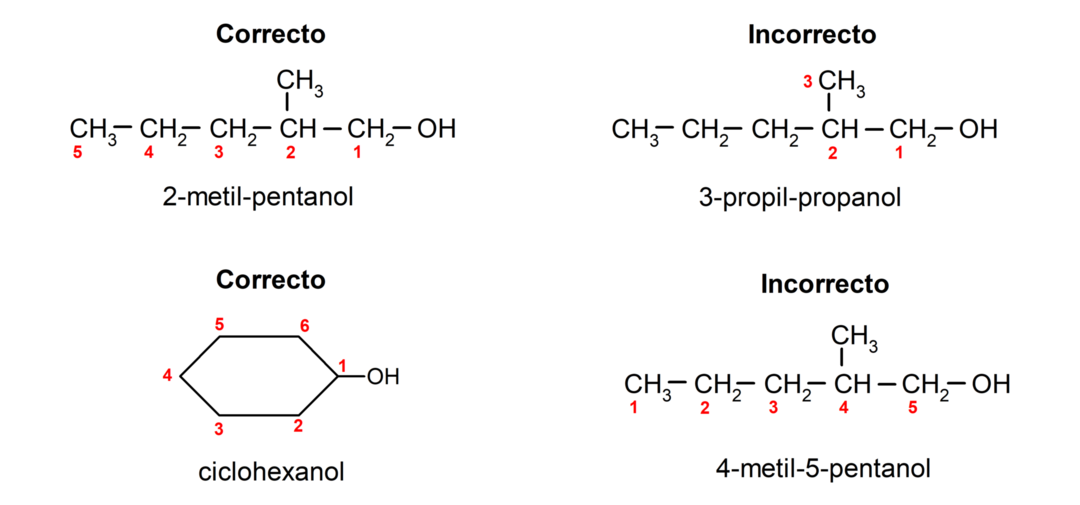
According to Traditional nomenclature, alcohols are named by writing the word “alcohol” and then writing the name of the alcohol. alkane corresponding to the carbon chain, but instead of using the -ane ending of the alkane, the ending -yllic.
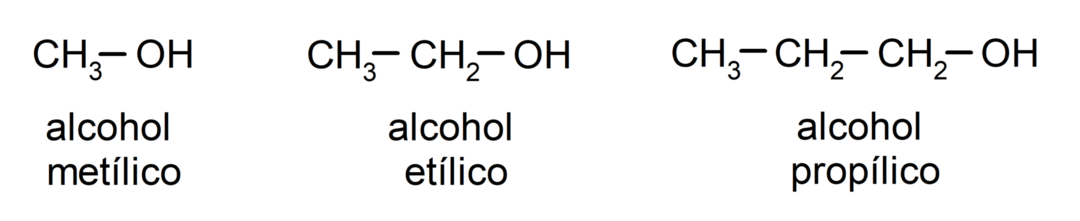
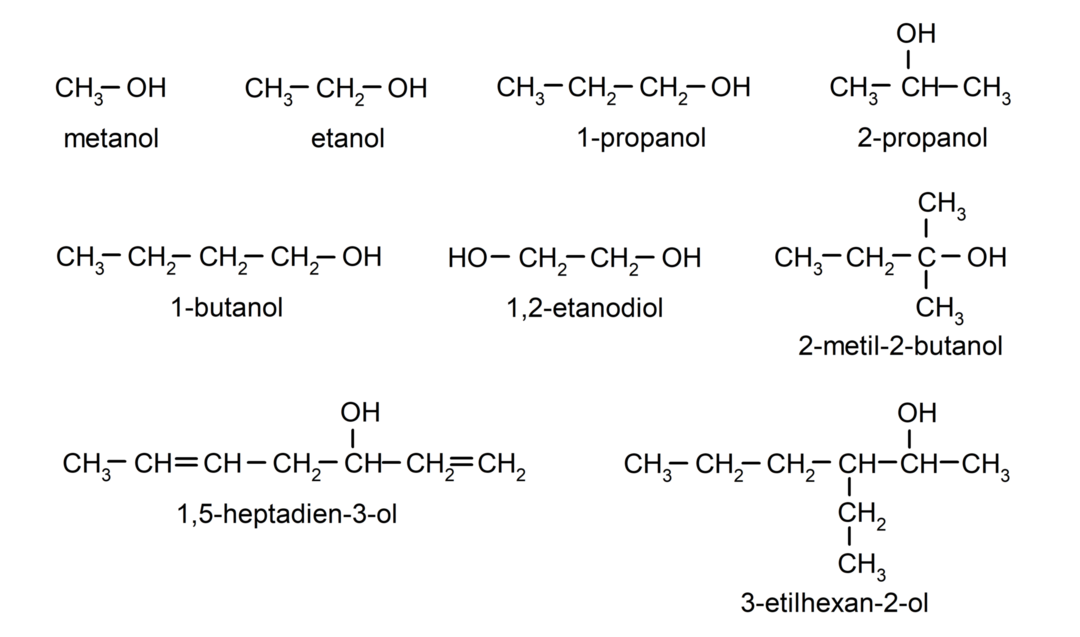
Examples of alcohols
- methanol
- ethanol
- 1-propanol
- 2-propanol
- 1-butanol
- 3-ethylhexan-2-ol
- glycerol
- phenol
- 2-methyl-2-butanol
- 1,2-ethanediol
- 1,5-heptadien-3-ol
- 3-methyl-2-butanol
- benzylmethanol
- 3-pentanol
- 2-methylphenol
- 1,2-dihydroxyphenol
- cycloheaxnol
- 1-phenylethanol

Uses of alcohols
The most common uses of alcohols are:
- They are used as disinfectants and antiseptics since they stop the growth of some microorganisms or destroy them. Ethanol and isopropyl alcohol are the most used for this purpose.
- They are used in the synthesis of different chemical compounds. Methanol, for example, is used to obtain methanal (formaldehyde).
- They are used to produce antifreeze. Methanol is one of the most used for this purpose.
- They are used as solvents in the pharmaceutical industry. Ethanol is one of the most used in this sense.
- They are used as solvents for lacquers, dyes and inks. Methanol is widely used for this purpose.
- They are used as fuel and there is an important tendency to partially replace the use of fossil fuels by the use of bioethanol fuel.
- They are used to make resins. Phenol is used in this sense.
Dangers of alcohol consumption
Although alcohol consumption is socially accepted today, the abuse of this substance generates dependence and addiction.
Ethanol is the alcohol present in alcoholic beverages, which when consumed in excess affects cognitive abilities, and can cause cardiovascular disease, liver cirrhosis and cancer.
Furthermore, when the degree of alcohol intoxication is reached, that is, a state of drunkenness, if the dose of the alcohol is too high, an alcoholic coma can occur, which can lead to respiratory paralysis and even death. death.
References
- T. TO. Geissman. (1974) “Principles of Organic Chemistry” Second Edition. Editorial Reverté, S.A. ISBN: 8429171800
- Ahumada-Cortez, J. G., Gámez-Medina, M. E., & Valdez-Montero, C. (2017). Alcohol consumption as a public health problem. Ra Ximhai, 13(2), 13-24.
- Morrison, R. T., & Boyd, R. N. (1998). Organic chemistry. Pearson education.
- Weininger, S. J., & Stermitz, F. R. (1988). Organic chemistry. I reversed.
Follow with:
- Ethyl alcohol
- Fuels
- organic chemistry



