30 Examples of Binary Sales
Examples / / November 09, 2023
The binary salts (neutral salts) are formed when a metal and a non-metal combine. Are chemical compounds made up of only two chemical elements. For example: sodium chloride (NaCl) and potassium bromide (KBr).
- See also: Metals and non-metals
Examples of binary salts
- Sodium chloride (NaCl)
- Iron(II) chloride (FeCl2)
- Iron(III) chloride (FeCl3)
- Cobalt(II) sulfide (CoS)
- Cobalt(III) sulfide (Co2Yes3)
- Lead(II) sulfide (PbS)
- Lead(IV) sulfide (PbS2)
- Calcium chloride (CaCl2)
- Sodium fluoride (NaF)
- Lithium chloride (LiCl)
- Strontium chloride (SrCl2)
- Barium chloride (BaCl2)
- Aluminum chloride (AlCl3)
- Magnesium sulfide (MgS)
- Rubidium chloride (RbCl)
- Calcium bromide (CaBr2)
- Potassium sulfide (K2S)
- Magnesium bromide (MgBr2)
- Zinc sulfide (Zn2S)
- Lithium bromide (LiBr)
- Nickel chloride (NiCl2)
- Uranium(III) chloride (UCl3)
- Silver bromide (AgBr)
- Silver iodide (AgI)
- Potassium bromide (KBr)
Nomenclature of binary salts
According to Traditional Nomenclature, binary salts are named by writing the name of the non-metallic element with the ending -ide. On the other hand, the name of the metallic element is written according to its oxidation state:
- For the lowest oxidation state, it is written with the ending -oso. For example: ferrous chloride (FeCl2), where iron has an oxidation state of 2+.
- For the highest oxidation state, it is written with the ending -ico. For example: ferric chloride (FeCl3), where iron has an oxidation state of 3+.
According to Systematic Nomenclature, binary salts are named by writing the name of the non-metallic element with a prefix that indicates the amount of atoms of this element in the compound. In addition, the ending -uro is placed in the name of the non-metallic element. Then, the name of the metallic element is placed. For example: magnesium dichloride (MgCl2) and iron trichloride (FeCl3).
According to Stock's nomenclature, binary salts are named by writing the name of the nonmetallic element with the ending -ide. Then, the name of the metallic element is placed followed by its oxidation state written in Roman numerals and in parentheses. For example: iron(II) chloride (FeCl2) and cobalt(III) sulfide (Co2Yes3).
Applications of binary salts
- They are used as refrigerants in the food and pharmaceutical industries. For example: calcium chloride (CaCl2).
- They are used to treat metal surfaces against corrosion. For example: sodium fluoride (NaF).
- They are used to regulate humidity in the paper and construction industry. For example: calcium chloride (CaCl2).
- They are used in the glass industry to eliminate impurities. For example: silicon tetrachloride (SiCl4).
- They are used as cooking ingredients. For example: sodium chloride (NaCl).
Physical properties of binary salts
- They have high melting points because they are made up of ionic bonds.
- They conduct electric current when dissolved or molten.
- The most common salts have low hardness.
- They are not compressible.
- Most can dissolve in water.
How are binary salts obtained?
Binary salts can be obtained through some of the following chemical reactions:
Reaction between a metal and a non-metal. For example: the reaction between sodium (Na) and dichloride (Cl2) produces sodium chloride (NaCl).
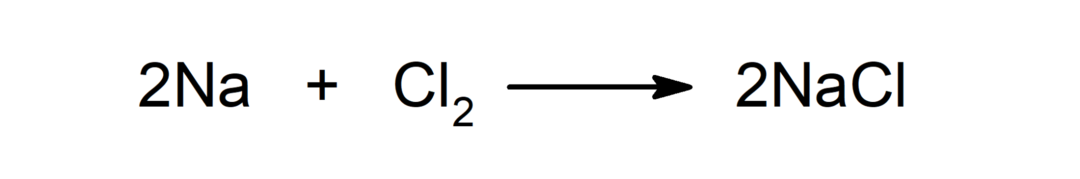
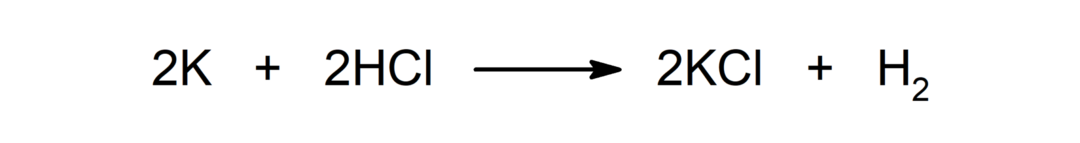
Reaction between a metal and an acid. For example: The reaction between potassium (K) and hydrochloric acid (HCl) produces potassium chloride (KCl) and dihydrogen (H2).
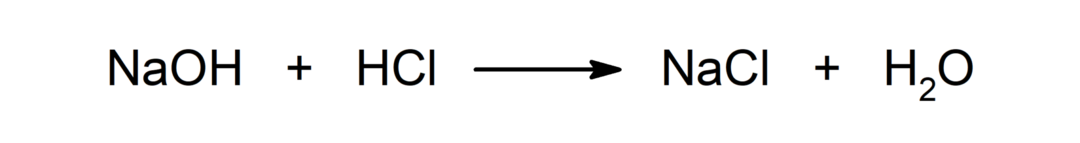
Reaction between an acid and a base. For example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2EITHER).
Follow with:
- oxysalts
- neutral salts
- acid salts
References
- Cerón Villalba, A., Novoa Ramírez, C. S., & Alpizar Juárez, E. (2020). Nomenclature video 1: binary salts and chemical reaction.
- Acurio Arias, M. V., & Delgado Méndez, M. AND. (2022). Game-based learning guide for “binary compounds” in High School Chemistry at the “Herlinda Toral” Educational Unit (Bachelor's thesis, National University of Education).
- Cabrera, M. J. H. (2005). Study from first principles of electronic and structural properties of binary and ternary compounds (Doctoral dissertation, University of La Laguna).
