25 Examples of Halides
Examples / / November 09, 2023
A halide or halide it's a chemical compound binary that is made up of a atom of a halogen and an element or cation that has a lower electronegativity than that of the halogen. For example:
- Sodium chloride (NaCl)
- Calcium fluoride (CaF2)
- Potassium bromide (KBr)
- Hydrogen chloride (HCl(g))
Halogens are chemical elements that make up group 17 of the Periodic table. The halogens are: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At), Teneso (Ts).
- See also: Aldehydes and ketones
Types of halides
Depending on their composition, halides can be:
- Inorganic halides. They are inorganic chemical compounds that contain one or more atoms of a halogen. They can be inorganic salts, hydrogen halides or metal complexes. For example: sodium chloride (NaCl) and the tetraiodomercurate anion HgI42-.
- Organic halides. They are organic chemical compounds that contain one or more atoms of a halogen bonded to a carbon atom of an organic compound. For example: methyl chloride (CH3 – Cl) and trichloromethane (CH – Cl3).
Examples of halides
- Sodium chloride (NaCl)
- Calcium chloride (CaCl2)
- Silver fluoride (AgF)
- Lithium fluoride (LiF)
- Potassium iodide (KI)
- Potassium bromide (KBr)
- Copper(II) chloride (CuCl2)
- Iron(III) chloride (FeCl3)
- Chromium(III) bromide (CrBr3)
- Aluminum chloride (AlCl3)
- Ammonium chloride ((NH4)Cl)
- Lead(II) iodide (PbI2)
- Magnesium iodide (MgI2)
- Beryllium fluoride (BeF2)
- Aluminum fluoride (AlF3)
- Bromomethane (CH3 – Br)
- Iodoform (CHI3)
- Methyl chloride (CH3 –Cl)
- Bromoethane (CH3 – CH2 – Br)
- Dichloroethane (Cl – CH2 – CH2 –Cl)
- Silver bromide (AgBr)
- Tin(IV) chloride (SnCl4)
- Titanium(IV) chloride (TiCl4)
- Hydrogen chloride (HCl(g))
- Hydrogen bromide (HBr(g))
Physical properties of halides
Some of the physical properties of halides are:
- Alkyl halides (organic halides) have higher densities and boiling points than their corresponding alkanes. This occurs because the halogen atom that has replaced a hydrogen atom in the hydrocarbon from which the halide comes.
- Organic fluorides and chlorides are less dense than water, while organic bromides and iodides are denser than water.
- Alkyl halides are insoluble in water and soluble in organic solvents.
- Hydrogen halides are gases with an intense odor at room temperature.
- Halides formed with the atoms of the elements in group 1 of the Periodic Table are white solids.
Chemical properties of halides
- Alkyl halides undergo aliphatic nucleophilic substitution reactions. These are reactions in which a nucleophilic atom (rich in electrons) replaces an atom (leaving group) that is attached to an electrophilic atom (deficient in electrons).
For example: in the reaction of methyl bromide (CH3 – Br) with sodium hydroxide (NaOH), the bromine (leaving group) is replaced by the hydroxide ion (nucleophile) in methyl bromide, where carbon is the electrophile.
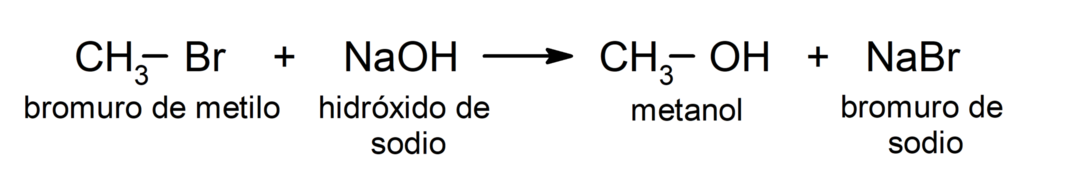
- Some alkyl halides react with magnesium in solvents such as ether or tetrahydrofuran, and the resulting product is an organometallic compound called the “Grignard reagent.” For example: ethyl bromide (CH3 – CH2 – Br) with magnesium (Mg) reacts in a dry medium to produce ethylmagnesium bromide (CH3 – CH2 – MgBr).

- Primary alkyl halides undergo reactions with alkoxides or alcohols in basic medium to produce ethers. These reactions are known as the “Williamson synthesis.” For example: the reaction between methyl bromide (CH3Br) and sodium methoxide (CH3ONa) produces dimethyl ether (CH3OCH3).
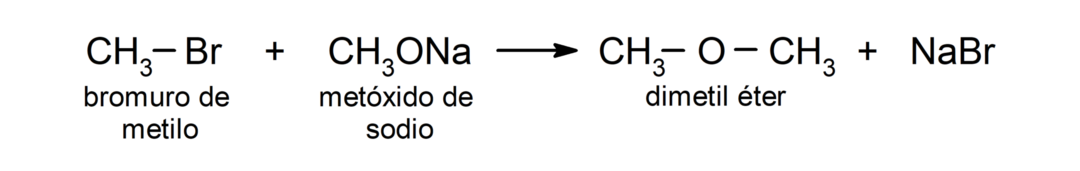
Uses of halides
Halides or halides have various applications in industry, medicine and daily life. Some of these applications are:
- They are used as solvents for many organic compounds.
- They are used as disinfectants and antiseptics.
- They have been used as refrigerants.
- They are used in the manufacture of fluorinated polymers. For example, Teflon.
- They are used as edible salts or tinctures in medicine.
- They are used in water treatment and purification.
- Metal halides are used in the manufacture of lamps.
- They are used in cleaning electronic components.
- They are used as starting chemicals to synthesize more complex organic compounds.
- Some, such as silver bromide (AgBr), are used in developing photographic plates.
Dangers of halides
Alkyl halides used as solvents react violently with strong bases and strong oxidants, which can cause explosions and fires.
Additionally, chlorofluorocarbons (CFCs) are alkyl halides that have been used for decades as refrigerants, but their Employment has been prohibited by international entities because they are mainly responsible for the hole in the layer of ozone.
On the other hand, hydrogen halides are irritating to the eyes, skin and mucous membranes.
Follow with:
- You go out
- acid salts
- neutral salts
References
- SALOMONS, T. g. (1996). Fundamentals of Organic Chemistry. Wiley.
- Whitten, K. W., Gailey, K. D., Davis, R. E., de Sandoval, M. T. TO. O., & Muradás, R. M. g. (1992). General chemistry (pp. 108-117). McGraw-Hill.
- Wells, A. F. (1978). Structural inorganic chemistry. Revert.



