Ionic Bond Example
Chemistry / / July 04, 2021
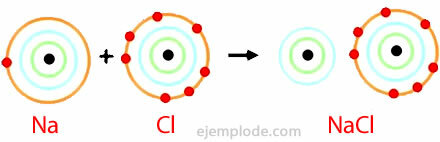
The ionic bond is given by the presence of a cation and an anion, chemical species with electrical charges of opposite signs. It is defined as the electrostatic force that binds ions in an ionic compound.
Atoms of elements with low ionization energies tend to form cations. In contrast, those with high electron affinity tend to form anions.
The alkali and alkaline earth metals are more likely to form cations in ionic compounds, and halogens and oxygen are the most likely to form anions. As a consequence, the composition of a great variety of ionic compounds results from the combination of a group IA or IIA metal and a halogen or oxygen.
For example, the reaction between Lithium and Fluorine produces Lithium Fluoride, a poisonous white powder that is used to lower the melting point of solder and in the manufacture of ceramics. The electron configuration of Lithium is 1s2, 2s1, and that of Fluorine is 1s2, 2s2, 2 P5. When these atoms come into contact, the valence electron 2s1 Lithium is transferred to the fluorine atom.
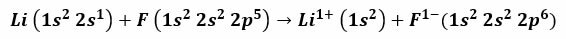
It is valid to assume that the procedure begins with the detachment of the lithium electron, ionizing this to reach the positive 1+ valence. It continues with the reception of this electron by Fluorine, which gives it a negative charge. In the end, the formation of the ionic bond occurs by electrostatic attraction. The Lithium Fluoride compound will be electrically neutral.
Many common reactions lead to the formation of ionic bonds. For example, the combustion of calcium in oxygen produces calcium oxide:
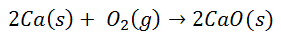
The diatomic oxygen molecule separates into two individual atoms. Then there will be a transfer of two electrons from the calcium atom to each oxygen atom. Both will then have their respective charges: for Calcium 2+ for each atom, and for Oxygen 2- for each atom. Upon final bonding, the Calcium Oxide molecule is electrically neutral.
Lattice Energy of Ionic Compounds
With the ionization energy and electron affinity values of the elements it is possible to predict what elements form ionic compounds, but it is also necessary to evaluate the stability of this type of compounds.
Ionization energy and electron affinity are defined for processes that occur in the gas phase, although all ionic compounds are solid at 1 atmosphere of pressure and 25 ° C. The solid state is a very different condition because each cation is surrounded by a specific number of anions and vice versa. Consequently, the overall stability of the solid ionic compound depends on the interactions of all the ions and not only on the interaction of a cation with an anion.
A quantitative measure of the stability of any ionic solid is its lattice energy, which is defined as The Energy necessary to completely separate a mole of a solid ionic compound into its ions in the gaseous state.
Born-Haber Cycle to Determine Lattice Energy
It is not possible to measure lattice energy directly. However, if the structure and composition of an ionic compound are known, it is feasible to calculate its lattice energy by applying Coulomb's Law, which states that the potential energy between two ions is directly proportional to the product of their charges and inversely proportional to the distance between them. To stop.
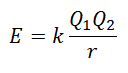
Since the charge of the Cation is positive and that of the Anion is Negative, the product will give a negative result in Energy. This represents an exothermic reaction. Consequently, to reverse the process, energy must be supplied.
It is also feasible to determine lattice energy indirectly if it is assumed that an ionic compound is formed in several stages. This procedure is known as Born-Haber cycle, which relates the lattice energies of ionic compounds with ionization energies, electronic affinity, and other atomic and molecular properties. This method is based on Hess's Law of Algebraic Sum of Chemical Reactions, and was developed by Max Born and Fritz Haber. The Born-Haber cycle defines the different stages that precede the formation of an ionic solid.

Sodium chloride
Sodium Chloride is an ionic compound with a melting point of 801 ° C, which conducts electricity in the molten state and in aqueous solution. Rock salt is one of the sources of sodium chloride and is found in underground deposits that are often several hundred meters thick. Sodium chloride is also obtained from sea water or from brine (a concentrated NaCl solution) by solar evaporation. Also, it is found in nature in the mineral called Halite.

Sodium chloride is used more than any other material in the manufacture of inorganic chemical compounds. The world consumption of this substance is about 150 million tons per year. Sodium Chloride is used mainly in the production of other inorganic chemical compounds, such as Chlorine gas, Sodium Hydroxide, Metallic Sodium, Hydrogen gas and Sodium Carbonate. It is also used to melt ice and snow on highways and roads.