Volumetric Analysis Example
Chemistry / / July 04, 2021
On Analytic chemistry, the Volumetry is an Analysis Method that consists of the Measurement of the Volume of Reagent needed to React Stoichiometrically with the Analyte, which is the substance to be determined.
React Stoichiometrically refers to the substances interacting chemically, according to the proportion that marks the chemical equation already balanced.
The substance to be investigated is called Analyte. From this substance, a known and exactly measured volume will be taken as a sample, which we will call Aliquot, contained in an Erlenmeyer flask to begin the analysis.
Concepts of Volumetric Analysis
Volumetric Analysis, being a type of Chemical analysis, carry involved a chemical reaction. This chemical reaction will be carried out between the sample with an unknown quantity of the investigated substance, and a known quantity of another substance, capable of reacting with the former.
The Reaction substances will be in aqueous solution, and the volumes of solutions that interacted to reach the end of the reaction will be measured, which will be visible thanks to an indicator that will give color inside the flask.
In Volumetric Analysis, the aqueous solution of Known Concentration is called Standard Solution, Standard or Titrant, and will serve as a reference for, ending the reaction, to make the calculation that will reveal the amount of the investigated substance.
This procedure of making both solutions react is called Chemical Degree o Valuation, which is the fundamental part of Volumetric Analysis. Consists of going gradually pouring the Standard Solution (Titrant) in the aliquot (Sample), until the indicator shows the change with a color difference.
A Indicator It is a chemical substance that is added to the Aliquot, showing a coloration, and that when the reaction ends, it will change color.
A Volumetric Analysis consists of a simple series of steps:
1.- Preparation of Standard Solution
2.- Preparation of the Sample or Aliquot
3.- Chemical Degree
4.- Volume Measurement
5.- Calculation of the investigated substance.
To the point where they have already fully reacted the two substances, it is called Equivalence point.
As in any chemical method of Analysis, in Volumetric Analysis there are requirements for good results:
-The chemical reaction must be Selective, that is, the standard solution will only react with the sample.
-The chemical reaction must be Stoichiometric, that is, obey the proportions marked in the balanced chemical equation.
-The chemical reaction must be Quantitative; refers to 99.9% completion at the equivalence point.
-There must be a detectable end point in reaction, which will be better confirmed by the indicator.
Primary Pattern
The Primary Patterns They are substances of high purity whose concentration in solution is calculated directly from the quantity weighed and the volume used of water.
A) Yes, can be reacted with working solutions, to know the concentrations of the latter and convert them into standard solutions.
Examples of Primary Patterns are:
-Sodium Carbonate (Na2CO3): It is used to standardize acids, such as Sulfuric Acid.
-Potassium Biphthalate: It is the primary standard to prepare standard solutions of Bases, such as Sodium Hydroxide.
-Sodium Chloride (NaCl): It is used to standardize Silver Nitrate solutions.
-Calcium carbonate (CaCO3): Primary standard for EDTA (Ethylene Diamine Tetraacetic Acid).
A Primary Pattern must meet several essential characteristics:
Must have a High Purity, Atmospheric Stability, Absence of hydration water, Low cost and easy to get, Y High equivalent weight.
Classification of Volumetric Analysis
Depending on the chemical species with which you work to make an analysis, it will be the type of Volumetry:
Acid-Base Volumetry: It works, either with an acid or with a base, to analyze samples that contain acids or bases.
Precipitation Volumetry: Also called Argentometry, it uses a standard solution of Silver Nitrate to determine how many Chlorides are in a sample.
Complexity Volumetry: A standard solution of a complexing agent, such as EDTA, is used to measure the concentration of Hardness, that is, Calcium and Magnesium Carbonates, in the Water.
REDOX volumetry: The reaction occurs between an oxidizing substance and a reducing substance.
Indicators
Of the numerous indicators used in Volumetrics, three stand out:
1.- Methyl orange: It takes an orange coloration in the aliquot, which will be titrated with an acid. When the equivalence point is reached, the indicator will turn yellow.
2.- Phenolphthalein: It is transparent in the beginning in the aliquot, which will be titled with a base. When the equivalence point is reached, the indicator will turn pink.
3.- Eriochrome Black: It is the indicator used to determine the hardness in water. In the beginning it is purple in the aliquot, until it is titrated with a complexing agent. At the end of the reaction, it turns blue.

Instruments in a Volumetric Analysis
In a Volumetric Analysis, a series of instruments will be used that, if they are not available, cannot be adequately developed:
1.- Volumetric Flask: It is a container like a bulb in its lower part, flattened at the bottom, which has a thin column in which the liquid is added. It has a mark to indicate where the meniscus of the liquid should be, so that it covers the exact volume. It is used to contain Standard Solutions; thanks to the accuracy of its volume, a known concentration is ensured.

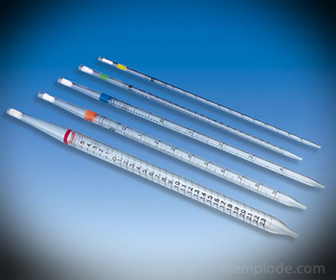
2.- Pipettes: They are graduated thin tubes that allow to measure reliably exact amounts of liquid. They are available up to 25 milliliters, and allow accurate sampling.
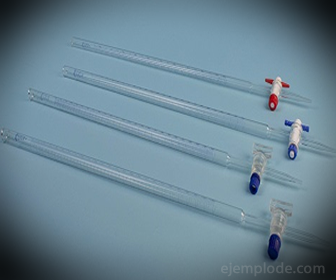
3.- Burette: It is an elongated tube with a capacity of 50 milliliters, which will contain the Standard Solution. At one of its ends it has a flow regulating valve that will drain the Solution into the Aliquot.
4.- Erlenmeyer flasks: They are containers with a flat base, conical shape that ends in a cylindrical edge. This design makes them suitable for chemical titrations, since they do not allow the stirring of the solutions to overflow them. A minimum of three will be required if repetitive testing is to be done, to ensure a reliable result.

Calculations in a Volumetric Analysis
As aqueous solutions are handled, the quantities of importance for a Volumetric Analysis are Concentration and Volume.
The main equation of Volumetry is based on four main data:
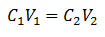
C1= Sample Concentration (unknown)
V1= Volume of the Aliquot, with which the analysis was worked
C2= Concentration of Standard Solution used to reach the equivalence point
V2= Volume Standard Solution needed to reach the end of the reaction
The data are substituted in the equation, leaving only the concentration sought as unknown. Of course, all the data must be on the same drives.
Volumetric Analysis Examples
Determination of Chlorides (Cl-) by Argentometry, with Silver Nitrate.
Determination of Bromides (Br-) by Argentometry, with Silver Nitrate.
Determination of Cyanides (CN-) by Argentometry, with Silver Nitrate.
Determination of Magnesium Carbonate (MgCO3), by Complexometry, with EDTA.
Determination of Calcium Carbonate (CaCO3), by Complexometry, with EDTA.
Sulfuric Acid Analysis (H2SW4) with Sodium Hydroxide (NaOH).
Analysis of Sodium Hydroxide (NaOH) with Hydrochloric Acid (HCl).
Determination of Antimony (III) with Potassium Permanganate (KMnO4).
Determination of Arsenic (III) with Potassium Permanganate (KMnO4).
Determination of Titanium (III) with Potassium Permanganate (KMnO4).
Determination of Molybdenum (III) with Potassium Permanganate (KMnO4).
Determination of Iron (II) with Potassium Permanganate (KMnO4).
Determination of Oxalate ion with Potassium Permanganate (KMnO4).
