Metallic Bond Example
Chemistry / / July 04, 2021
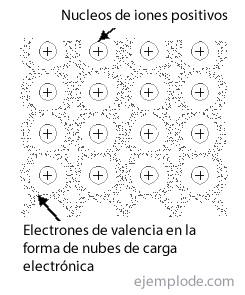
The Metallic Link is the way in which the atoms of a metal manage to meet in a crystal structure, stable, using the clouds of its electrons, and without depending on the exclusivity of ionic or covalent bonds to manifest as pure substances. Is named Net to the group or arrangement of metallic links.
Most metals crystallize in three types of arrangements: Body Centered Cubic Networks, Face-Centered Cubic Networks Y Compact Hexagonal Networks.
In the of body centered, each atom of the metal is surrounded by 14 neighbors, and in the others two remaining by 12. If you try to deal with the bonding of such structures, you will immediately find the problem of electron insufficiency. Thus, in the case of Lithium with a single valence electron and 14 close neighbors, it must be explained how this element is surrounded by such a large number of atoms, and yet it generates a crystal stable enough to have a melting point of 186 ° C. The same happens with other metals.
The Swiss physicist Felix bloch In 1928 he proposed a quantum mechanical theory to explain the bonding of atoms in metallic crystals. In this
Band Theory all electrons present in an atom at fully filled energy levels are considered essentially located, that is, bound to the atoms to which they are associated. On the other hand, valence electrons in unfilled energy levels are considered free, and they move in a potential field that extends to all the atoms present in the crystal.The atomic orbitals of these free electrons in an atom can overlap with those of others to originate delocated molecular orbitals that produce a bond between all the atoms present, and that are known by the name of Orbitals of Conduction.
The energy levels of electrons in isolated atoms are discreet and generally well spaced. But the presence of other atoms in the crystal affects these levels by transforming each level into a level band whose number is equal to the number of atoms present in the structural totality. If this number is large, each isolated level constitutes practically a continuous band. Also, when the space between the original levels and between the atoms in the metal is large, then bands originating from early electronic levels are separated from each other for energy gaps considerable. When levels and distances are small, the bands are cross and overlap each.
This theory provides the following description of the electronic structure of a given metal. A solid metal is considered to have electron bands separated from each other by energy gaps. Furthermore, these bands are sometimes totally filled with localized electrons, or they are partially filled with free electrons whose molecular orbitals extend to all atoms in the crystal.