Aromatic Compounds Example
Chemistry / / July 04, 2021
Chemists have found it useful to divide all organic compounds into two broad classes: compounds Aliphatic and compounds Aromatics. Aromatic compounds are Benzene and compounds with similar chemical behavior. The aromatic properties are what distinguish Benzene from Aliphatic Hydrocarbons. The benzene molecule is a ring of a particular type. There are other compounds, also ring-shaped, that appear to differ structurally from benzene and yet behave in a similar way.
It turns out that these other compounds resemble benzene in their basic electronic structure, which is why they also behave as aromatics.
Aliphatic hydrocarbons (alkanes, alkenes, alkynes and their cyclic analogues) react mainly by addition, in multiple links, and by free radical substitution, at other points in the aliphatic chain.
On the other hand, aromatic hydrocarbons, it is emphasized that they have the tendency to heterolytic substitution. Furthermore, these same substitution reactions are characteristic of aromatic rings wherever they appear, regardless of what other functional groups the molecule might contain. These latter groups affect the reactivity of aromatic rings, and vice versa.
The benzene molecule
Benzene has been known since 1825, and its chemical and physical properties are better known than those of any other organic compound. Despite this, it was not until 1931 that a satisfactory structure had been proposed for this substance, and it took up to 15 years for it to be in general use among Chemicals organic. The difficulty lay in the limitations of the development that structural theory had reached by then. The final structure has been reached thanks to the assumption of several important facts:
Benzene has the molecular formula C6H6. Due to its elemental composition and molecular weight, benzene was known to have six Carbon and six Hydrogen atoms. The problem was knowing the arrangement of such atoms.
In 1858, August Kekulé proposed that Carbon atoms can be linked together to form chains. Later, in 1865, he offered an answer to the benzene problem: these carbonate chains can sometimes be closed, to form rings.
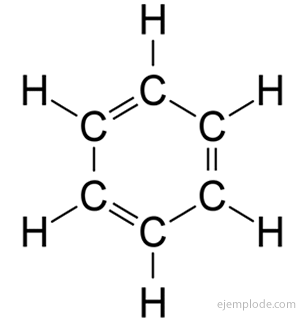
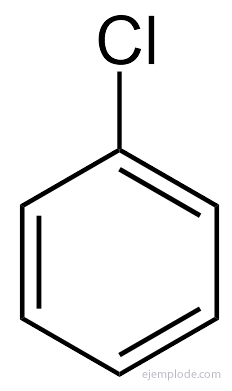
Benzene only gives a monosubstituted product C6H5Y. For example, when a hydrogen atom is replaced by Bromine, only a single configuration of BromoBenzene C is obtained.6H5Br; analogously, a ChloroBenzene C is also obtained6H5Cl, or a NitroBenzene C6H5NOT2, etc. This fact imposes a severe limitation on the structure of Benzene: all its Hydrogen must be exactly equivalent, that is, they must all be joined to Carbons that in turn are all equally linked. There can be no hydrogens in CH3, and others in CH2, and others in CH. The final structure of the monosubstituted should be the same for the substitution of any Hydrogen in Benzene.
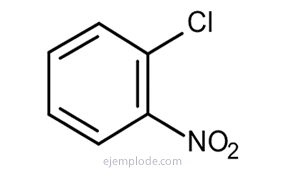
Benzene gives three isomeric disubstituted products, C6H4Y2 or C6H4AND Z. There are only three isomeric DiBromoBenzenes, C6H4Br2, three ChloroNitroBenzenes C6H4ClNO2, etc. This fact further limits the structural possibilities.
Benzene undergoes substitution reactions, rather than addition reactions. Kekulé's benzene structure corresponds to one that we would call Cyclohexatriene. Because of this, it should easily react by addition, as do the similar compounds, cyclohexadiene and cyclohexene, which is a characteristic of the structure of alkenes. But that is not the case; under conditions where alkenes react rapidly, benzene does not react, or only very slowly. Instead of addition reactions, benzene easily undergoes a set of reactions, all of which are substitution, as the Nitration, the Sulfonation, the Halogenation, the Friedel-Crafts alkylation, the Acylation from Friedel-Crafts. In each of these reactions, an atom or group has been replaced by one of the Hydrogen atoms of Benzene.
The stability of Benzene is due to the alternating double bonds and also to the resonance energy, in the one in which the double bonds change their position between the carbons, maintaining the same alternation structural. Is resonance stabilization energy is responsible for the set of properties called Aromatic Properties.
An Addition reaction turns an alkene into a more stable saturated compound. But in the case of Benzene, an Addition makes it less stable by destroying the ring system sustained and stabilized by resonance. The final molecule would be Cyclohexadiene. It is because of this fact that the stability of Benzene leads it to only Substitution reactions.
Properties of Aromatic Compounds
In addition to substances that contain benzene rings, there are many others that are considered aromatic, although on the surface they hardly bear any resemblance to Benzene.
From an experimental point of view, aromatic compounds are substances whose molecular formulas suggest a high degree of unsaturation, despite which they are resistant to addition reactions so characteristic of unsaturated compounds.
Instead, these aromatic compounds a often undergo electrophilic substitution reactions similar to those of Benzene. Along with this resistance to addition, and probably because of it, there is evidence of a unusual stability, such as low heats of hydrogenation and combustion.
Aromatic substances are cyclical, usually presenting rings of five, six, and seven atoms, and their physical examination shows that they have flat or nearly flat molecules. Its protons have the same type of chemical shift in Nuclear Magnetic Resonance spectra as in Benzene and its Derivatives.
From a theoretical point of view, for a substance to be aromatic, its molecule must have cyclic clouds of delocalized π electrons above and below the plane of the molecule; Furthermore, these π clouds must contain a total of (4n + 2) π electrons; this means that delocalization is not enough for the particular stability that characterizes an aromatic compound to result.
Nomenclature of Benzene Derivatives (Aromatic Compounds)
In the case of many of these derivatives, especially in monosubstituted ones, it is enough to prepend the name of the substituent group for the word Benzene, such as, for example, ChloroBenzene, BromoBenzene, IodoBenzene, Nitrobenzene.
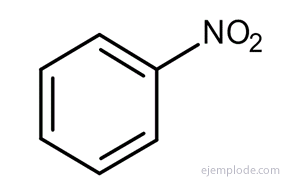
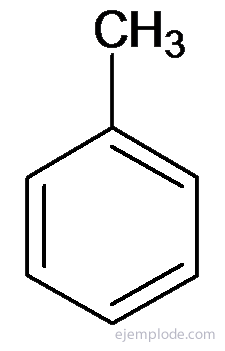
Other derivatives have special names that may lack similarity to the name of the substituent group. For example, Methyl Benzene is only known as Toluene; AminoBenzene as Aniline; Hydroxybenzene as Phenol, etc.
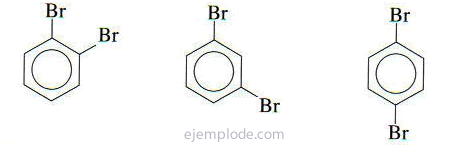
If there are two groups attached to the benzene ring, it is not only necessary to identify which they are, but also to indicate their relative location. The three possible isomers for disubstituted benzenes are characterized by the prefixes ortho, meta and para, abbreviated o-, m-, p-. For example: o-DiBromoBenzene, m-DiBromoBenzene, p-DiBromoBenzene.
If one of the two groups is of the type that gives the molecule a special name, the compound is named as a derivative of that special substance. For example: NitroToluene, Bromophenol, etc.
Examples of Aromatic Compounds
Toluene or Methylbenzene
Ethylbenzene
Isopropylbenzene
TriNitroToluene or TNT
Aniline or Aminobenzene
Benzoic acid
Glutamic Acid or ParaAminoBenzoic Acid
Toluene Sulphonic Acid
Phenol or Hydroxybenzene
Bromophenol
Trichloro benzene
Benzene Phenyl Ether
Iodine benzene
Bromo benzene