Dalton's Law Example
Chemistry / / July 04, 2021
The English scientist John Dalton (1766-1844) was a physicist and chemist who made many contributions to the compression and classification of the elements and chemicals. Among his main contributions is the first model of representation of atoms and compounds through a graphic representation. He also formulated the so-called "Law of Multiple Proportions" also known as Dalton's Law.
On chemical combinations, Louis Proust (1754-1826) formulated the "Law of constant proportions", in which he collects the observation that chemicals always combine in the same proportion to produce the same compounds. That is, if hydrogen and oxygen are combined, they will always combine in the same proportions, to produce water. However, this law did not explain how there were different compounds made up of the same substances.
Using his atomic theory, Dalton realized that when some simple substances are combined, they can different compounds are produced and that the amounts of one of the substances vary in a proportionate proportion simple.
Dalton's Law is stated like this: The weights of an element that join with the same amount of another to form different chemical compounds vary according to a very simple relationship.
This means that if we know how substances are combined in simple proportions, and the amount of one of the substances remains constant and another substance we put in a simple proportion, as a ratio of 2, 3 or 4 in relation to the original proportion, in each case we will obtain a substance different. This happens for example, when combining phosphorus, hydrogen and oxygen.
If we combine 1 volume of phosphorus, 3 volumes of hydrogen and 2 volumes of oxygen, we will obtain hypophosphoric acid:
P + 3H + 2O -> PO2H3
If in the previous reaction we vary only the oxygen in a simple proportion of 2, we will obtain phosphoric acid:
P + 3H + 4O -> PO4H3
It must be taken into account that at this time the concept of atomic valence was not yet known, so it was not known exactly why some substances could be combined in variable proportions and others not.
Dalton's Law Example
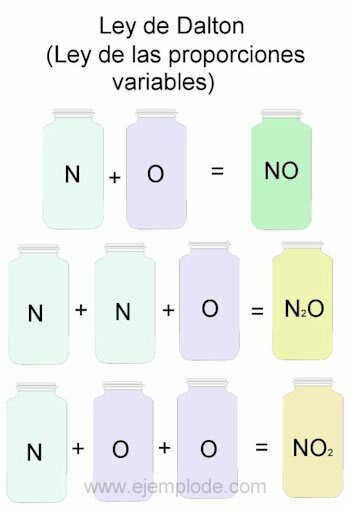
To exemplify Dalton's Law, we will take as a reference the combinations of Nitrogen (N) and oxygen (O).
When they are combined in the same proportion, that is, one by one, we will get nitric oxide:
N + O -> NO
If we keep the nitrogen volume constant and vary the oxygen by 2, we will obtain nitrogen peroxide:
N + 2O -> NO2
If, on the basis of nitric oxide, we now keep oxygen constant and vary the volume of nitrogen by 2, we will obtain nitrous oxide:
2N + O -> N2OR
If the article was useful to you, do not forget to give us a +1.



