Example of Chemical Elements
Chemistry / / July 04, 2021
A chemical element is a pure substance made up of atoms of the same type, in turn made up of a number of protons and neutrons in the nucleus, and a number of electrons in their orbitals. Some are found naturally, others by forming the molecules of a compound, and others have been created through the execution of laboratory procedures. All chemical elements make up the matter of the entire universe, and sustain the existence and functioning of the human body.
Each element has characteristics and behaviors that give it a distinction and make it unique, but at the same time it presents similarities with a group of elements in the manifestation that they have in the universe. For this reason, there is the Periodic Table of Chemical Elements, which addresses these similarities to coherently group the elements, and make it easier to study their properties.
Atomic number of an Element
The Atom carries a number of protons in the nucleus, accompanied by the same number of neutrons. This number is called the Atomic Number, represented as
Z for academic purposes and in Literature. For each Element, this number will be unique. There are no two Elements with the same atomic number. The Periodic Table also focuses on this criterion for ordering them.Symbol of an Element
In the times of Alchemy, which span approximately the year 400 to 1000 AD. C., the Alchemists recorded their experiments assigning symbols to the elements. They were simple symbols, made with geometric figures, to represent the individual element and the transformations studied.
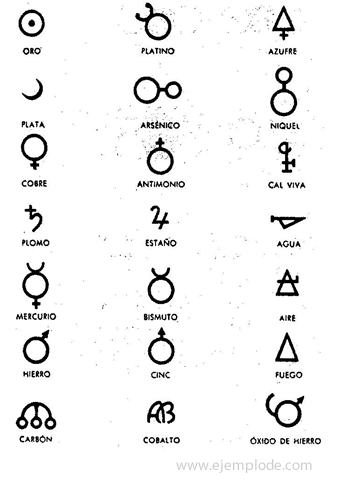
Some examples of alchemical symbols, with the name of the element or compound they represented.
In the Era of Modern Chemistry, the Elements are still represented with symbols, in this case letters that refer to their name in Latin or English.
Examples of Chemical Symbols:
The symbol for Sodium is Na, by its Latin name Natrium
The symbol of Gold is Au, by its Latin name Aurum
The symbol for Silver is Ag, by its Latin name Argentum
The symbol for Copper is Cu, by its Latin name Cuprum
The symbol of Antimony is Sb, by its Latin name Stibium
The symbol of Iron is Faith, by its Latin name Ferrum
The symbol of Mercury is Hg, by its Latin name Hydrargyrum, which means "liquid silver"
The symbol for Potassium is K, by its Latin name Kalium
Chemical Element Groups
The Periodic Table of Chemical Elements classifies these by groups: groups A and groups B. The A groups are eight, which contain the Alkaline Elements, the Alkaline-Earth elements, the Earth elements, three families of Elements, whose head defines the name of the family: Carbon Family, Nitrogen Family, Sulfur Family, Halogens and Gases Nobles. Groups B are made up of all transition metals and Rare Earths, which are also two large Families: Lanthanides and Actinides.
Group IA: Alkaline Elements
The series of alkaline elements is made up of Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr). They all have an electronic configuration such that in the last shell they have an electron. Their name is due to the fact that when they come into contact with water, they react to form Alkalis or Hydroxides. It is a general behavior between these elements. The larger the atom of the element, the more reactive it is, since the force with which the nucleus retains the electron from the last shell has less and less reach. They are capable of forming Ionic Bonds with Halogens. For example: The ionic bond that has an abundant presence, is that of Sodium-Chlorine, forming Sodium Chloride NaCl.
Group IIA: Alkaline-Earth Elements
These elements are: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). They are not free in nature; instead, its carbonates and silicates exist in relative abundance. They are Argentine white and crystalline. They easily combine with oxygen if exposed to air. This is due, in part, to the fact that they have two electrons in their last shell, consistent with the oxygen-receiving capacity. Barium is the group's most active element, and together with Calcium, they are the ones with the most industrial applications in the group.
Group IIIA: Earth Elements
Group IIIA comprises the elements Boron (B), Aluminum (Al), Gallium (Ga), Indium (In) and Thallium (Tl). Boron is a non-metallic element, Aluminum is amphoteric (amphiprotic), that is, it is capable of acting as an acid and as a base; and the other three are metallic elements. They have three electrons in their last shell, generating a valence of +3, although sometimes Gallium acts with a +1 and +2 valence in some of its compounds. Boron is the only element in this series that forms Hydrides. Boron and Aluminum form Carbides.
Group IVA: Carbon Family
The representative elements of group IVA are Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn) and Lead (Pb). The first two are fundamentally non-metallic in their characteristics, but Germanium, Tin, and Lead are metallic, and the more so the higher their atomic number. With the exception of Silicon, each element has the valences +4 and +2.
Carbon and Silicon form compounds in which atoms of the elements are joined by pairs of shared electrons. Carbon is the essence of Organic compounds by associating with atoms of Hydrogen, Oxygen, Nitrogen, Sulfur, and sometimes Silicon.
Silicon and Germanium are used for the manufacture of electronic components, as they have the property of behaving like semiconductors.
Group VA: Nitrogen Family
Group VA comprises the elements Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb) and Bismuth (Bi). Nitrogen and Phosphorus are non-metallic, Arsenic and Antimony are metalloids, and Bismuth is a metal. These elements are characterized by forming Hydrides, of which the least toxic is Ammonia NH3. Nitrogen forms Nitric Acid HNO3, involved together with Hydrochloric Acid in Agua Regia, a mixture capable of dissolving precious metals such as Gold and Silver.
Nitrogen is also involved in two large groups of organic compounds called Amines and Amides, which can be considered derivatives of Ammonia NH3, by substitution of a Hydrogen by a Hydrocarbon chain.
Group VIA: Oxygen Family
Made up of the elements Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te) and Polonium (Po). Oxygen is the most active, and has the ability to easily form covalent bonds. In contact with metals in a high humidity environment, it forms oxides. It forms a resonant molecule called Ozone, which protects the planet from UV radiation.
Group VIIA: Halogens
Its name means "Sales Formers". The group is made up of the elements Fluor (F), Chlorine (Cl), Bromine (Br), Iodine (I) and Astate (At). They have seven electrons in the last shell, which allows them to be receptors for one electron. This quality makes them link with the elements of group IA, forming binary salts. Fluor is characterized by having the largest Electronegativity of the entire Periodic Table, with a value of 4.0, its counterpart being Cesium, with electronegativity of 0.7. This property allows it to have the strength to attract other atoms and give priority to form a bond with they.
Group VIIIA: Noble Gases
Also called the Inert Gas Group, it is made up of the elements Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Ra). They are the elements that have all their complete electronic configuration, so they are not capable of reacting under usual conditions. They are used mainly for luminous commercial signs, emitting visible light when an electric current is incident on them.
Groups B: Transition metals
In ten groups of three elements each, the transition metals are grouped. These include the best electrical conductors: Silver (Ag), Copper (Cu), Gold (Au); the best structural components for urban construction and engineering; Iron (Fe), Titanium (Ti), Aluminum (Al), Zirconium (Zr), Tungsten (W); the best catalyst components: Nickel (Ni), Vanadium (V), Platinum (Pt); and the main coating ingredients: Cadmium (Cd), Chromium (Cr), Zinc (Zn). They usually handle valences between +1 and +3, but elements like Chromium handle valences +2, +3, +6.
Rare Earths: Lanthanides and Actinides
They are called Rare Earths for their scarcity on the planet. They are made up of two groups: Lanthanides and Actinides. They are found on the two separate lines of the Periodic Table. They work with a valence of +3 generally, and tend to form hydroxides. The most important element among them is Cerium, which is used in the preparation of pyrophoric alloys (Alloy Mischmetal, for stones for lighters), in gas hoses for lighting and in the manufacture of special glasses that absorb ultraviolet rays and heat radiation.
The most powerful radioactive elements, such as Uranium (U) and Plutonium (Pu), are part of these groups. that given their instability, release energy and disintegrate, losing Alpha particles (nuclei of Helium). Later they become less unstable elements, according to the radioactive series.


