Example of Reactive Materials
Chemistry / / July 04, 2021
The Reactive Materials are those substances that when in contact with some other, tend to start a chemical reaction. At the end of this reaction, a certain amount of different substances is the result.
Reactivity in substances
The Reactivity is the capacity of substances interact with others in a chemical reaction. All the participants in this reaction will be called Reactive. When the chemical phenomenon ends, different substances called Products.
In the industrial environment, Reactivity is used when you want generate a quantity of a product wanted. What is done in industrial plants is to choose the raw material that has the appropriate Reagents in its composition, place it in a container called Reactor for the chemical reaction to take place, and make sure there is an economical process and efficient.
There are times when the reaction it will be spontaneous, and it will be carried out effortlessly, just by having the reagents in contact. It would only be required to shake the reagents to favor their incorporation.
On other occasions, the simple contact of the Reagents will not suffice, but it will be necessary to modify the conditions of the process, either adding more agitation to the Reactor, or introducing heating, or even cooling, so that the reaction originates and proceeds with better speed and yield.
The Reagents will start to get involved to generate Products, but they will according to the proportion that indicates the equation that expresses the reaction, as in the following:
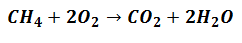
It means that Methane Reagents (CH4) and Oxygen (O2) will be mixed in a ratio of 1 to 2 molecular units to form the corresponding products.
If one of the Reagents is added in least amount which is required as calculated, will be called Limiting Reagent, because when everything is consumed, the reaction will stop.
The other Reagent, which at the end of the reaction will be a surplus, will be called Reactive in Excess, and of course it will come together with the Products.
The Reactivity of substances can be used for many chemical phenomena, such as Oxidations, in the case of Potassium Permanganate; Catalysis, as in the case of Platinum metal in presentation of a fine mesh; elemental exchanges, as in the case of zinc metal, which replaces hydrogens in acids.
Reactivity as a property of Hazardous Materials
The Reactivity represents a risky property when the reactive material is already a waste without use, so it is first poured into a container that ensures that it remains isolated from human contact. When the waste is solid or liquid, it is usually thick plastic containers, which carry as a mandatory feature a sticker with the pictogram of "Reagent Material", which is an image with an orange background and the corresponding symbology in black. The following image shows the variety of signals for the different types of Reactivity.
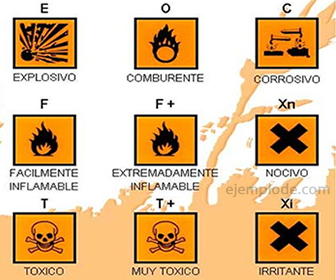
Reactivity is one of the properties CRETIB of Hazardous Waste: Corrosive, Reactive, Explosive, Toxic, Flammable and Biological-infectious. In fact, it is the most representative, because all the other properties have to do with a mode of reactivity.
A very frequent case in which the danger of these materials is exposed, is when a spill of these occurs. For example, when a Sulfuric Acid leak occurs and it spills over a very large area, personnel who do not have the training to control the situation should be removed. People trained in this regard must intervene.
The procedure to eliminate the dangerous situation begins by going to the site, wearing the appropriate personal protective equipment. The area of the Reactive material leak is identified and isolated, and its Reactivity will begin to counteract with another chemically opposed species. In this case, Sulfuric Acid acts as an acid. It will be possible to extinguish this reactivity only with a species such as Sodium Hydroxide, one of the most powerful bases.
The reaction between an Acid and a Base is called Neutralization. With the help of this phenomenon, the reactivity of the Sulfuric Acid in the leak will be directed towards the activity of the Sodium Hydroxide.
There will come a time when Sodium Hydroxide, also a reactive material, ends the dangerousness of Sulfuric Acid. When the reaction is over, it will only be a matter of diluting the products with an abundant flow of water.

Examples of Reactive Materials
- Sodium hydroxide
- Calcium hydroxide
- Magnesium hydroxide
- Sulfuric acid
- Hydrochloric acid
- Nitric acid
- Hydrogen Sulfide
- Potassium nitrate
- Sodium bicarbonate
- Carbon dioxide
- Sulfur dioxide
- Sulfur trioxide
- Hydrogen gas
- Gaseous oxygen
- Chlorine gas
- Gaseous bromine
- Metallic lithium
- Metallic sodium
- Metallic cesium
- Metallic magnesium
- Ammonium nitrate
- Sodium chloride
- Potassium chloride
- Calcium chloride
- Potassium permanganate
- Sodium Permanganate
- Metallic platinum
- Metallic zinc
- Calcium Carbide
- Acetylene
- Methane
- Ethane
- Propane
- Butane



