Organic Chemistry Example
Chemistry / / July 04, 2021
The Organic chemistry It is the part of General Chemistry in charge of the study and functional classification of chemical compounds whose structural element is the Carbon, present in abundance in the living matter; thus a well-differentiated universe of Inorganic Chemistry is created, dedicated to chemical compounds classified as minerals.
The Carbon atom has four electrons in the last shell of its electronic configuration. This characteristic, together with that of its atomic radius, allows it to be joined by covalent bonds to other atoms of the same element, in numerous chains, forming a great diversity of chemical compounds stable.
You can check Covalent bonds.
Such chemical compounds, in addition to the Carbon atoms that give them their primordial structure, contain Hydrogen atoms as the main complement for the Carbon valence. In addition, in this diversity of compounds, there are those with the intervention of oxygen atoms, Nitrogen, Halogens, Sulfur and even Phosphorus, alkaline and alkaline earth elements and metals of transition. Depending on who is involved in the molecule, it will be the physical and chemical properties of the final substance.
The organic compounds are present throughout the living matter; promote and sustain all biological functions, unlike inorganic or mineral substances, which have been used by humans for commercial purposes, for experimentation or to fulfill certain domestic tasks.
History of Organic Chemistry
Before 1828, the distinction was already being made between Organic Chemistry and Inorganic Chemistry. Organic matter was associated with a creation through a "vital force", and to the inorganic with the non-living, with the mineral. Given these conceptions, one could not expect to obtain organic compounds from inorganic materials in the laboratory.
However, in 1828 Friedrich Wöhler (1800-1882) succeeded in preparing an organic substance, Urea CO (NH2)2, an important product of animal metabolism, from inorganic compounds. Treatment Lead Cyanate Pb (CNO)2 with Ammonia NH3 to get Ammonium Cyanate NH4CNO; This compound was formed, indeed, but when the solution was boiled to crystallize the Ammonium Cyanate, it was transformed into Urea.
This chemical change is an example of Internal regrouping, in which the number or class of atoms in the molecule does not change, but only their order within it. These types of transformations are very common in organic chemistry.
Wöhler's discovery initiated the abandonment of the life force theory, later completely discarded by being followed by the preparation of many other organic compounds in the laboratory. Despite this, the qualifiers of Inorganic and Organic subsist, since inorganic compounds are related to mineral products and organic compounds, which are actually compounds of carbon and hydrogen and their derivatives, are of the type produced by living organisms.
Although the laws of general chemistry apply equally to both compounds, various causes justify and make this division necessary. Thus, organic and inorganic compounds differ in different properties, such as: their Solubility preferred in organic solvents (ether, alcohol, chloroform, etc.) and in water, respectively, its Stability (organic compounds decompose at relatively low temperatures), and the Nature of Reactions; for inorganic compounds they are ionic, simple and practically instantaneous, and for organic compounds they are covalent, complex and slow.
Organic compounds
Carbon compounds with chains of up to ninety atoms are known. The chains Carbon atoms can be linear and branched and have single covalent bonds or have double or triple bonds. More than 2,500 compounds are known to contain only Carbon and Hydrogen (Hydrocarbons).
Isomerism of organic compounds
In inorganic compounds, a formula generally represents a single compound; thus, there is only one substance of formula H2SW4. The Sulfuric Acid molecule contains two Hydrogen atoms, one of Sulfur and four of Oxygen, in a specific and unique arrangement. In organic compounds it is rare for this to happen. Thus, for example, there are two compounds that respond to the formula C2H6Or, Ethyl Alcohol or Ethanol, and Dimethyl Ether.

The more complex the molecule, that is, the greater the number of Carbon atoms, the greater the number of possible isomers.
Organic Compound Analysis
The analysis of an organic compound comprises the qualitative analysis, the quantitative analysis and the Functional analysis. In the case of supposing that the compound is in an impure state, it is previously purified by Crystallization, Distillation, Sublimation, Extraction, etc. The purity criterion can be judged based on its physical constants, such as melting point, boiling point, density, solubility, crystalline form, refractive index, etc.
The qualitative analysis It is verified by investigating the presence of the elements that make up the compound, especially Carbon, Hydrogen and Nitrogen, and sometimes Halogens, Sulfur and Phosphorus.
The quantitative analysis It is carried out using as a basis the methods used for qualitative analysis. The starting point is a certain quantity of substance that undergoes combustion, and the carbon dioxide is collected and weighed. carbon and water vapor formed to calculate the percentage of Carbon and Hydrogen in the compound. The results of the quantitative analysis facilitate the calculation of the empirical formula, although the molecular formula can only be found after the determination of the molecular weight of the substance. But the problem is not yet solved, because the same molecular formula can correspond to different isomers.
Classification of Organic Compounds
According to their structure, organic compounds are divided into compounds aliphatic, aromatic Y heterocyclic. Aliphatic compounds are related to methane CH4, are open chain, except for cycloparaffins, and owe their name to the fact that animal and vegetable fats belong to this group.
The aromatic compounds, closed chain, closely related to benzene, C6H6, and they owe their name to the fact that many of them have fragrant, pleasant smells.
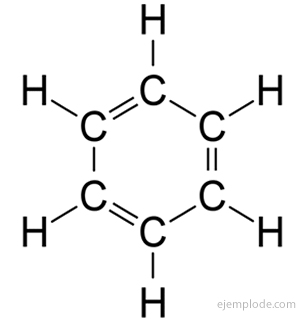
Benzene Ring
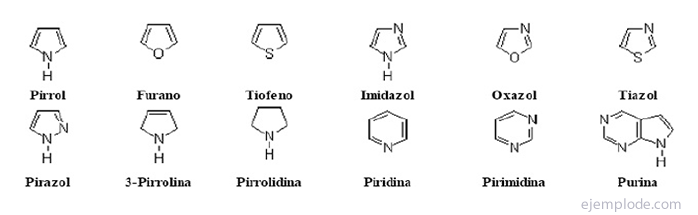
The heterocyclic compounds They are closed chain compounds in which there is an element other than carbon in the ring.
By their constitution, they are:
Hydrocarbons, which in turn, due to the type of links it has, is classified as Alkanes, Alkenes Y Alkynes. In addition, Cycloalkanes, Benzene and their derivatives only made up of Carbon and Hydrogen are in this category.
Heterocyclic compounds
Halogenated derivatives:R-X
Nitro compounds:R-NO2
Sulfonic Acids:R-SO3H
Nitriles (OR alkyl cyanides) and Isonitriles:R-CN and R-NC. They differ in how the Nitrogen atom is bonded in the molecule.
Alcohols:R-OH
Phenols: Compounds based on a backbone consisting of a benzene ring with an added hydroxyl group.

Ethers:R-O-R
Mercaptans: R-SH
Thioethers:R-S-R
Thioacids:R-COSH
Aldehydes:R-CHO
Ketones:R-CO-R
Carboxylic acids:R-COOH
You go out:R-COOM (M for metal)
Esters: R-COO-R
Anhydrides: R-CO-O-OC-R
Amines:R-NH2, R-NH-R, 2R-N-R
Aldosa type carbohydrates:-CHOH-CHOH-CHO
Ketose-type carbohydrates:-CHOH-CO-CH2Oh
Organometallic compounds:R-M-R
Metal-Alkyl Halides:R-MX (also known as Grignard Reagents)


