Corrosive Substances Example
Chemistry / / July 04, 2021
To understand the nature of corrosive substances, it is essential to know the phenomenon of Corrosion, which is the degradation of a material by chemical action of environmental agents such as oxygen in the air, or substances such as Acids and Bases.
A Corrosive Substance is that gas or liquid that has the tendency to corrode or destroy the surface with which it comes in contact.
Corrosion is notable, for example, in Metals. By constant contact with water and oxygen in the air, the mass of metal is converted, at a speed that varies depending on the metal element, into Oxide of the same, which gives the impression that the main material is physically destroyed.
Corrosion is dangerous if it occurs on surfaces like human skin. The contact of the corrosive substance with mucous membranes, tissues and organs is highly destructive, to the point of causing irreversible damage. It is called Chemical burn to the result of such a contact, because the corrosive substance acts at the molecular level.
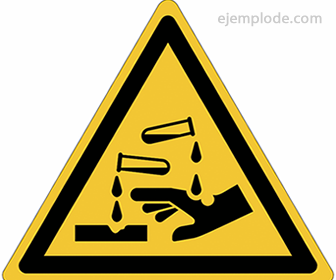
Corrosive Substances Pictogram
For the identification of a corrosive substance and to take into account accident prevention measures and personal protective equipment, a universally usable image is used, called Pictogram, consisting of a rhombus filled in one color, and a black image that explains the effect of the substance in question.
The tanks to contain corrosive substances are generally made of opaque plastics that do not allow the content to be seen. Standardized labels are produced in accordance with the Official Mexican Standard NOM-018-STPS-2000, which specifies the system for the identification and communication of hazards and risks due to hazardous chemical substances in the workplace.
Security measures
If a person is going to handle or transport any corrosive substance, It is essential to put on the Personal Protective Equipment first. Such equipment generally, for the purpose of handling corrosives, consists of a full-body, one-piece plastic suit with a respirator containing the appropriate cartridges for such environments, thick plastic gloves and rubber boots that reach up to the calf. In addition, safety glasses are required that cover the face as well as possible. If possible, a mask can be used.
With Personal Protective Equipment contact and potential accidents with the corrosive substance are avoided; The confidence with which it is handled is improved and the maneuver is more successful.

To store corrosive substances in large volumes, containers with a limited variety of materials are used. Those materials that are not greatly affected by the action of corrosive substances are stainless steel. (only for not so large quantities) and polypropylene, for example. Polypropylene has a firm structure that is not overcome by corrosion.

For its storage a specialized infrastructure is required, which in the event of a leak or spill represents a barrier so that the substance does not disperse on the ground.
Correct signaling of the network is required for its transport through pipelines, in accordance with the previously mentioned Mexican Official Standard NOM-018-STPS-2000. The valves must be in the best sealing condition to avoid any leakage or spillage.
Examples of Corrosive Substances
Hydrochloric acid
Hydrochloric Acid is the strongest Hydracid, given its activity in solution. When it is at concentrations of 1 M (1 mol / liter) it already emits vapors that are irritating to the nose of those who inhale them directly, and if it comes into contact with the skin begins to itch, then a growing burning that will make it red, which means that there will already be tiny wounds, imperceptible to the simple sight.
Perchloric acid
Perchloric Acid is an oxyacid derived from Hydrochloric Acid. Thanks to the oxygen in its molecule, the corrosive power of this substance is much greater. It is very aggressive with human tissues.
Sulfuric acid
Sulfuric Acid is an oxyacid resulting from the reaction between Sulfur Trioxide and Water. Since its equilibrium humidity is higher than the ambient humidity, or in other words, it is hygroscopic, has a tendency to dehydrate the surface on which it comes in contact, if it contains some Water. This is where its corrosive effect begins. On human skin it also generates an initial burning, the degree of which depends on how dilute or concentrated the acid is.
Regia Water
Agua Regia is a mixture of two acids, Hydrochloric Acid and Nitric Acid. It is so corrosive that it is capable of dissolving precious metals, such as Gold (Au) and silver (Ag). The joint effect of these acids is what achieves it. Each one fulfills its specific function: Hydrochloric Acid is responsible for, through its atoms of Chlorine that get involved in the solution, detach the Gold atoms, generating soluble complexes such as Au (Cl4)-. Meanwhile, Nitric Acid is placing its Nitrate NO group3 in the available places, preventing the Gold from returning to the initial structure of the metal.
Sodium hydroxide
Sodium Hydroxide, known as Caustic Soda for its corrosive characteristic, is a strong base to whose contact the human skin reacts with a stinging burning. Due to its ease of ionization, it is used in solution for the rapid neutralization of acid leaks. Another application that Sodium Hydroxide Solution has is to break down the fats that solidify in domestic stoves and ovens.
Potassium hydroxide
Potassium Hydroxide, known as Caustic Potash for the same corrosive characteristic, is a strong base, with a slight weakness, compared to sodium hydroxide. It is used for the saponification of fatty acids, in the soap production process, and also in solution to alkalize the medium in which the reagents interact in a specific reaction.
Nitric acid
Nitric Acid has the peculiarity that its vapors do not keep rising when they emerge from the container, rather, they escape to slide on the surface that surrounds the container, as if they were overflowing it. Nitric Acid is generally used for mixing with Hydrochloric Acid to produce Aqua Regia, and as a reagent to introduce a Nitro group (NO2) to organic compounds such as Benzene C6H6, to have products such as TriNitroToluene (TriNitroMetilBenzene).
More examples of corrosive substances:
Hypochlorous Acid (HClO)
Chlorous Acid (HClO2)
Chloric Acid (HClO3)
Acetic Acid (CH3COOH)
Hydrogen Sulfide (H2S)
Hydrofluoric Acid (HF)
Fluoroantimonic Acid (HSbF6)
Ammonium Hydroxide (NH4OH)
Butyl Lithium (C4H9Li)
