Surface Tension Example
Physics / / July 04, 2021
The Surface Tension is the Force exerted on the surface of a liquid that is at rest, to counteract the weight of a light object resting on it. Furthermore, the Surface Tension can be defined as the Force exerted by a liquid to resist being broken on its surface. It is the main property that holds soap bubbles, consistent and firm.
Liquids have as one of their main properties a fixed volume, whose shape will vary according to the container that contains them. Liquids adapt to the shape of the container, always occupying the lowest part of it by gravity. In this way, they leave a free surface, not totally flat, or adopt special shapes such as drops, bubbles or bubbles.
There are then Surface Forces, calls Cohesion and Adhesion, which are explained below:
Cohesion: It is when two different liquid surfaces are affected by a force of attraction to each other, which is activated in the outermost molecules.
Accession: It is when a liquid is incorporated by attraction to the surface of a solid, diffusing in that area.
These two Surface forces are responsible for various biological phenomena, based on the concepts of Surface Tension and Capillarity.
Surface tension
In a liquid, each molecule is surrounded by more molecules; in this way the attraction in all directions is compensated at every point of the liquid, except on the surface, where there are no molecules above, but only air, forces are directed into the liquid, thus generating a net attraction towards that content.
The liquid, then, tends to make cohesion, which is the same as not to disperse, and to minimize its surface, forming drops. The surface of the liquid will have the behavior of a film that offers resistance to its deformation, and therefore resistance to breaking.
To measure this Cohesion Force, a wire structure with a sliding side is considered, in which a layer of liquid is placed. A simple comparison of this structure is with a soap bubble blowing ring, in which you can slide the wire a little to make the ring bigger.
The liquid will try to minimize the surface, designated by S, exerting a force F on the sliding side, which can be measured. It is concluded that the calculation of the Force remains:
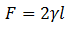
Where γ is the Surface Tension, and l is the length of the cable that slides.
The Surface Tension γ is a property of the liquid. The Force F will depend on l, the length of the sliding cable, but not on the surface S. The factor 2 is introduced because there are two surfaces, which are the internal and the external one of the sliding wire, which is in contact with that of the liquid.
The Surface Tension γ is the Force per unit length exerted by a surface of a liquid on any line, located on it as a clamping edge.
The Force originating from the Surface Tension is perpendicular to the surface line, and tangent to it.
The Surface Tension γ can also be defined as the Energy per Unit Area that is needed to increase an area, and is expressed with the following formula:
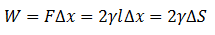
Since energy is required for a surface to form, liquids tend to reduce their exposed area relative to the surroundings. It is for this matter that the surfaces of bodies of water such as lakes, seas and oceans in a calm state are flat.
Surface Tension is measured in Newton each meter (N / m), and for each substance it decreases with increasing temperature. That of water is greater than in most liquids, and is also due to the fact that it is one of the densest liquids, with 1 g / cm3 density.
Below is a table with the values of some substances, typical of a series of temperatures.
Liquid |
T (° C) |
γ (N / m) |
Helium |
-270 |
0.0002 |
Hydrogen |
-255 |
0.002 |
Neon |
-247 |
0.005 |
Oxygen |
-193 |
0.016 |
Ethanol |
20 |
0.022 |
Soapy water |
20 |
0.025 |
Water |
100 |
0.059 |
Water |
60 |
0.062 |
Water |
20 |
0.073 |
Water |
0 |
0.076 |
Mercury |
20 |
0.465 |
Silver |
970 |
0.800 |
Surfactants or Surfactants
Sometimes it is required to reduce the Surface Tension of a liquid. Is achieved dissolving in it the substances called Surfactants or Surfactants, which form a surface film whose molecules are barely attracted by the molecules of the inner liquid.
Thanks to surfactants, it is easier for the liquid in question to get wet.
Examples of Surface Tension
The mosquito that lands on the water, remaining suspended on the surface.

A Styrofoam or Styrofoam plate suspended in water.

The sheet of water that forms on the ring before blowing a soap bubble.

The lightest particles of sand or dust, if not agitated, remain suspended on the surface of the water.

When there is water and oil in a glass, there is surface tension in the separation between the two by density.

When a flavored drink is shaken a lot, the bubbles generated remain on the surface, each one involved in the total surface tension.

The suds of soapy water in the operation of a washing machine have bubbles and bubbles that form before rinsing.

Ships take advantage of this property of water to stay afloat, thanks to the fact that they carry air inside. They are like a floating bubble on the surface of the water.

Surfboards create surface tension when the water is at rest, and when there is movement, they hold firm.

When raw milk is boiled, the generation of a cream begins, which is consolidated when the milk cools. It is a thick layer of fat on top of the liquid part.

Do not forget to leave your comments.