Definition of Chemical Nomenclature
Miscellanea / / July 04, 2021
By Javier Navarro, in Jan. 2016
 In nature as a whole there are more than a hundred different chemical elements, specifically 118. Chemical elements are a type of matter and are made up of atoms of the same kind. In the science of chemistry a table is used to classify and order the different elements, the known periodic table, in which the name of each element appears along with its respective abbreviation or symbol, as well as other data of interest (atomic number and atomic mass). To refer to the elements and to be able to combine them with each other, a type of denomination and a language specific, that is, a chemical nomenclature.
In nature as a whole there are more than a hundred different chemical elements, specifically 118. Chemical elements are a type of matter and are made up of atoms of the same kind. In the science of chemistry a table is used to classify and order the different elements, the known periodic table, in which the name of each element appears along with its respective abbreviation or symbol, as well as other data of interest (atomic number and atomic mass). To refer to the elements and to be able to combine them with each other, a type of denomination and a language specific, that is, a chemical nomenclature.
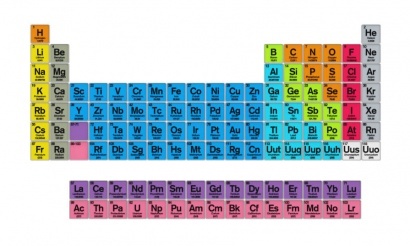
Classification of the periodic table
Metallic elements appear on the left side and in the center of the table, non-metallic elements appear on the side. right and the metalloids also on the right but forming a broken line.
The table is divided into 18 different vertical groups, because each line of elements has many characteristics in common. Likewise, the table presents 7 different levels.
When observing the periodic table it is appreciated that the elements are ordered from least to greatest in relation to their atomic number.
How to read the chemical nomenclature
To name an element of the periodic table an abbreviation is used with the big letters in the center of its corresponding box (for example, it is written He and in the lower part the full name of it is written, Helium). In the upper left corner of the element box is its atomic number, which in the case of helium would be 2. In the right corner we find the number of its atomic mass (4 in helium).
From the reading of a chemical element it is now possible to handle a nomenclature that allows reading the combination of the different elements. There are three different ways to name chemical elements: systematic, stock, and traditional, being the systematic nomenclature the most used, since it is the simplest.
 If we take the systematic nomenclature as a reference, it consists of using a series of prefixes depending on the number of atoms (mono, bi, di, tetra, etc). Thus, if in the molecule chemistry there is an atom we will use the prefix mono. A well-known example is CO2 or carbon dioxide (here the prefix di refers to the two oxygen atoms).
If we take the systematic nomenclature as a reference, it consists of using a series of prefixes depending on the number of atoms (mono, bi, di, tetra, etc). Thus, if in the molecule chemistry there is an atom we will use the prefix mono. A well-known example is CO2 or carbon dioxide (here the prefix di refers to the two oxygen atoms).
As for the stock nomenclature, it consists of putting the valence of the chemical element in parentheses and in Roman numerals, for example FeH2 is an iron (ll) hydride. The traditional nomenclature is probably the most complicated and consists of following a table with prefixes and suffixes depending on the oxidation number of the chemical element (the prefixes hypo or per and the suffixes bear and ico). For example, hypochlorous acid (Cl2O) or nickel sulfate (NiSO4).
Photos: iStock - Dvougao / Nerthuz
Topics in Chemical Nomenclature
