50 Examples of solutions
Miscellanea / / July 04, 2021
The solutions are one of the types of mixtures that exist. The components that make up a solution do not react chemically, although the physical properties of these components can be modified when they become part of the solution. For example: smoke, amalgam, coffee with milk.
For a mixture to be a solution, it must be homogeneous Y uniform, that is, that the mixed components cannot be distinguished with the naked eye and that, in addition, the proportion between the solute (a substance that appears in less quantity) and the solvent (substance that appears in greater quantity) remains approximately unchanged in whatever volume is taken from the solution. The proportion of the solute in the solution or in the solvent is what is called "concentration" and usually the same solution can be prepared using various concentrations of solute.
Solutions can form between substances that, before being mixed, are in any of the different aggregation states. There are solutions in practically all states of aggregation. Generally, the state of aggregation of the solution is determined by the state of aggregation of the solvent. For example:
It is common for presence of molecules of solute within a solvent alter the properties of the solvent itself. For example, the melting and boiling points of two compounds change when these compounds are mixed, as can their composition. densities and colors.
The French Scientist Roult studied this behavior of the components in solutions and also proposed its main law (Roult's Law), which states that the partial vapor pressure of each component in the vapor mixture surrounding an ideal solution from liquids it is equal to the partial pressure of each pure component times its mole fraction in the solution. An ideal solution is considered to be a solution in which the chemical species are very similar, so no variation in the energy of the interactions between them is considered. The fundamental equation of Roult's Law is:
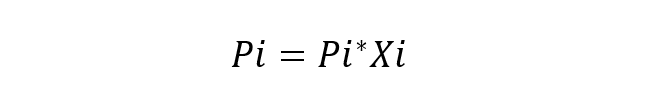
Where:
- Pi is the partial pressure of the component i in the gaseous mixture surrounding the solution.
- Pi* is the pressure of the component i
- Xi is the mole fraction of the component i in dissolution.
Obviously, people are permanently in contact with solutions. The air is a dissolution of elements in gaseous state: its majority composition is given by the nitrogen (78%) and the rest is occupied by 21% of oxygen and 1% of other components, although these proportions may vary slightly.
Examples of solutions
The following list includes forty examples of solutions, highlighting the state of aggregation in which each one is found, a solute in a respective solvent.
- Air (gas in gas). A composition of gases, where nitrogen is the most abundant.
- Smoke (solid in gas). The air is stale by the appearance of smoke from the fire. It is a solution in which air acts as a solvent.
- Alloys between metals (solid in solid). Duralumin is an alloy composed of aluminum, copper, manganese, magnesium, and silicon.
- Atmospheric air dust (solid in gas). The presence of solids (decomposed almost to an indivisible unit but finally solids) in the gas is an example of dissolution in this sense.
- Steel (solid in solid). Alloy between iron and carbon, with a much higher proportion of the former.
- Carbonated drinks (gas in liquid). Carbonated drinks have a dissolution of gases in a liquid.
- Amalgam (liquid in solid). They are alloys of mercury dissolved in certain metals like gold or silver.
- Refined petroleum (liquid in liquid). The combination of the elements that compose it (the majority is carbon) gives rise to a dissolution between liquids.
- Butane in air (gas in gas). Butane is a chemical compound gaseous that can be stored in tubes, ready to be used as fuel.
- Oxygen in ocean water (gas in liquid). The oxygenation of seawater allows the development of aquatic life.
- Drinks with an alcohol content (liquid in liquid). They are very consumed by humans in celebrations. They are usually solutions of ethanol and fruit juices in controlled concentrations of the alcohol.
- Coffee with milk (liquid in liquid). A liquid with a higher content receives a little from another, which represents a transformation of its color and flavor.
- Smog (gases into gases). The introduction of gases that are not typical of the atmosphere induces a transformation of the air, which it has negative effects on the societies that breathe it: the more concentrated, the more harmful it will be.
- Salt in water (solid in liquid). Widely used for cooking.
- Blood (liquid in liquid). The majority component is plasma (liquid) and within it other elements appear, among which the red blood cells stand out.
- Ammonia in water (liquid in liquid). This solution (which can also be made from a gas to a liquid) is functional to many cleaning supplies.
- Air with traces of humidity (liquid in gas). Water vapor is present in the air due to increased temperature.
- Powder juices (solid in liquid). The powder dissolves in water and produces a solution the color of powdered juice.
- Hydrogen in palladium (gas in solid). Hydrogen dissolves very well in some metals.
- Airborne Viruses (solid in gas). Like atmospheric dust, they are very small units of a solid that are transported by a gas.
- Mercury in silver (liquid in solid). It is one of the so-called “amalgams”.
- The tea (solid in liquid). A solid in very small dimensions (the granites of the envelope) dissolves on the water.
- Royal water (liquid in liquid). It is a composition of acids that allows dissolving different metals, among which gold appears.
- Bronze (solid in solid). It is the alloy between copper and tin.
- Lemonade (liquid in liquid. Although many times the mixture is between a solid and a liquid, it is actually a liquid present in that solid, such as lemon juice.
- Peroxide (liquid in liquid). It is a solution of hydrogen peroxide (H2OR2) in water. It is used to disinfect wounds and in the cosmetic industry.
- Brass (solid in solid). It is the alloy between the solid copper and zinc.
- Ice cooling (solid in liquid). Ice enters the liquid and cools it, while dissolving. If it is introduced in water, it is the particular case in which it is the same substance.
- Physiological solution (liquid in liquid). Water acts as a solvent and many liquid substances act as a solute.
- Smoothies (solids in liquids). Through a grinding process, a combination of solids to liquids is induced.
Follow with:
