Example of Chemical Bases
Chemistry / / November 13, 2021
In General Chemistry, Bases are a category of chemicals that fulfill several functions:
-React with Acids in a Neutralization, producing a Salt and Water.
-Regulate Hydrogen potential, pH, raising its value, if they are poured in the middle where a reaction is found.
-Run as Chemical reagents for a large number of reactions.
The Bases can be different species, both of the Inorganic chemistry as of the Organic chemistry, Like the Hydroxides, the Amines and the Alcohols, for example.
To define the behavior of the Bases, the three main Acid-Base Theories are used: Arrhenius's, Brönsted-Lowry's and Lewis's.
The Bases according to Arrhenius
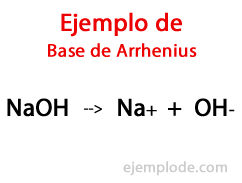
According to the Swedish Chemist Svante Arrhenius, Bases are those chemical substances that provide hydroxyl ions (OH-) to a reaction medium, especially if it is an aqueous solution. Thus, with the available hydroxyl ions, there will be a Alkaline pH, that is, with a value greater than 7, and up to 14 depending on how much Base is present at the time of measurement.
This is one of the simplest and most practical theories to apply, since it does not have complications to differentiate the substances that take part in the reaction. It is well known which is the Acid and which is the Base.
Within this Theory, there are indisputably inorganic Hydroxides, such as Sodium Hydroxide (NaOH) and Potassium Hydroxide (KOH).
The Bases according to Brönsted-Lowry
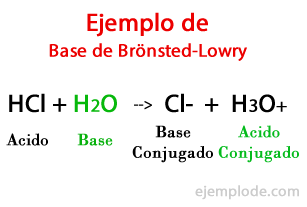
According to the theory formulated by the Danish Johannes Brönsted and the british Thomas Martin Lowry, a Base is a Chemical species capable of receiving the Protons that another, the Acid, is going to give during a chemical reaction. The proton is generally related to the positive charge that characterizes it, so we can associate it again with Hydrogen ions (H +).
When the exchange occurs in the chemical reaction, the products are called: "Conjugated Acid Base", and "Conjugated Acid Base", based on the reactants that formed them.
For this Theory, the Ammonia (NH3) is the most representative case. For Brönsted and Lowry, the substances that are capable of Retaining Hydrogen ions (H +) are Bases. In this case, the Ammonia, by behaving as a Base, will acquire a Hydrogen in its structure, consolidating itself as an Ammonium ion (NH4+), with the excess positive charge of Hydrogen. Ammonium is the Conjugated Acid of Ammonia.
Amines, organic compounds derived from Ammonia (NH3), such as Methyl Amine (CH3NH2), when in solution they behave as Bases, and are capable of receiving positive charges in their structure, either from Hydrogen or Carbocation.
A Carbocation is an organic ion formed as a hydrocarbon chain, which in the absence of a negative ion, which It can be the Hydroxyl (OH-) or a Halogen (Cl-, Br-), it tends to bind to a site that can receive it, which will be the Base.
The Bases according to Lewis
The American Scientist Gilbert lewis pointed out in his Acid-Base Theory that the Bases are those substances that are capable of contributing their pairs of free electrons for another to complete its octet.
This Acid-Base Theory is a complement to reaffirm the validity of its Rule of the Octet, in which it describes how atoms acquire stability by reaching a number of eight electrons in their last shell, using the Bond Covalent
The Hydroxyl ion is a good example of a Lewis Base. It has a pair of free electrons on which a Hydrogen ion that does not have electrons can reach. A molecule of Water will form. Thus the octet for the oxygen of the molecule will be formed, and the hydrogens, which are smaller atoms, will have their pair of electrons that will make them stable.

Uses of Important Bases
The Sodium Hydroxide NaOH It is generally used in aqueous solution to clean solidified grease in domestic and industrial stoves, dissolving it effectively. It is also used, in concentrated solution, when unforeseen spills of an acid substance occur, to neutralize it.
The Magnesium Hydroxide Mg (OH)2 It is used in a solution called "Milk of Magnesia", to solve heartburn, neutralizing it. It is sold in pharmacies.
The Potassium Hydroxide KOH It is used as a reagent for Saponification processes, transforming fats into Soap.
The Ammonia NH3 It is used in its gaseous form as an industrial refrigerant, especially in ice makers. It is very dangerous to use, since breathing a concentration of 5 milligrams per liter of it in the air can be fatal.
Examples of Chemical Bases
Water H2OR
Ammonia NH3
Sodium Hydroxide NaOH
Potassium Hydroxide KOH
Magnesium Hydroxide Mg (OH)2
Calcium Hydroxide Ca (OH)2
Aluminum Hydroxide Al (OH)3
Ammonium Hydroxide NH4Oh
Ferrous Hydroxide Fe (OH)2
Ferric Hydroxide Fe (OH)3
Hydroxyl Ion (OH-)
Chloride Ion (Cl-)
Bromide Ion (Br-)