30 Examples of Aldehydes
Examples / / November 06, 2023
The aldehydes are organic compounds which are formed by a carbon chain that has a terminal carbonyl group (= C = O) attached (located at one end of the molecule), which in turn is bonded to a hydrogen atom.
Very common aldehydes are methanal (formaldehyde), ethanal (acetaldehyde) and propanal (propaldehyde).

Some of these compounds are present in nature, for example, vanillin or vanillin is a natural aldehyde that constitutes the main flavoring of vanilla.
- See also: Aldehydes and ketones
Nomenclature of aldehydes
Aldehydes can be named using the nomenclature rules established by the International Union of Pure and Applied Chemistry (IUPAC).
To name an aldehyde, prefixes are used that indicate the number of carbon atoms that the carbon chain has attached to the carbonyl group. The carbonyl group is always located at one end of the carbon chain, which means that it will always have position one and it is not necessary to indicate its location in the chain. Additionally, the suffix -al is placed at the end of the aldehyde name. Some examples are:
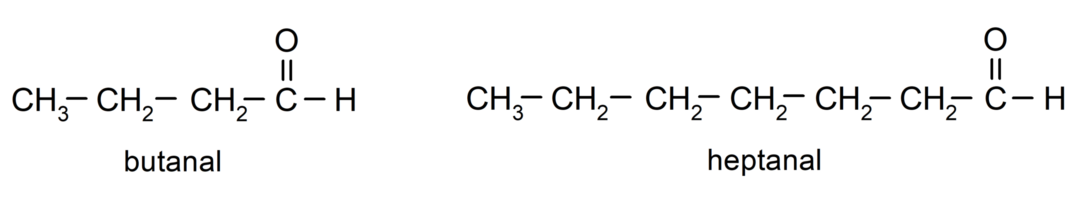
If the aldehyde is branched, that is, it is made up of several carbon chains, the main chain as the chain that has the greatest number of carbon atoms, and that also contains the group carbonyl. The rest of the chains are named as substituent groups.
Furthermore, each substituent group must be chosen so that its position occupies the lowest possible numbering in the main carbon chain. On the other hand, the carbon atoms of the main chain begin to be counted at the end that contains the carbonyl group. Some examples are:
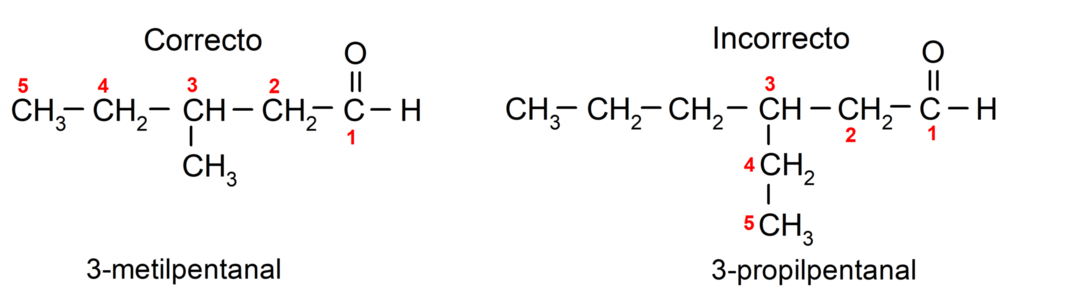
If the aldehyde acts as a substituent in the molecule, because it is also made up of groups higher priority functionals, such as acids and esters, then the aldehyde group is named as -oxo. For example:

If an aldehyde has several carbonyl groups, it is named using prefixes that indicate the number of these groups, dial (two carbonyl groups), trial (three carbonyl groups), etc. For example:
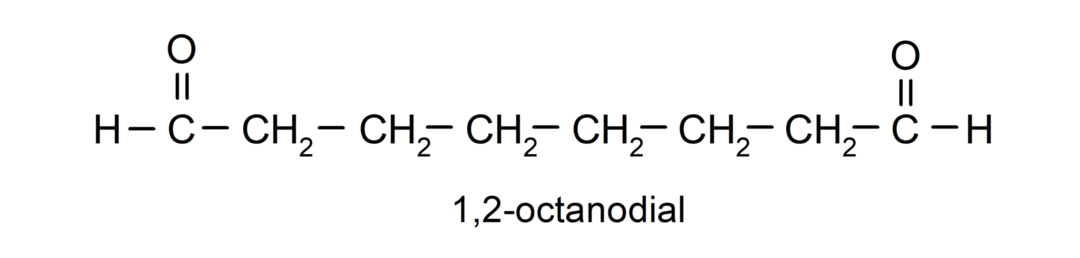
When the carbonyl group is linked to a cycle, aldehydes are named using the term carbaldehyde. For example:

Physical properties of aldehydes
Aldehydes usually have some variation of the same physical property. This occurs because the physical properties of aldehydes depend on how the carbon chain that is attached to the carbonyl group is formed.
Some physical properties of aldehydes are:
- Solubility. The water solubility of aldehydes depends on the amount of atoms that has the carbon chain. Aldehydes with shorter carbon chains (up to about five carbon atoms) are soluble in water. Aldehydes with carbon chains of many carbon atoms are not soluble in water. Methanal and ethanal are very soluble in water.
- Density. In general, aldehydes are compounds that are less dense than water.
- Aggregation States. Aldehydes made up of one and two carbon atoms are gases, those containing between three and twelve carbon atoms are liquids, and those made up of more than twelve carbon atoms are solid.
- Smell. Some aldehydes have irritating odors, while others have pleasant odors.
- Polarity. The carbonyl group gives them polarity.
- Boiling point. They have higher boiling points than alkanes of similar molecular weight, and they have lower boiling points than carboxylic acids and alcohols of comparable molecular weight.
Chemical properties of aldehydes
Some of the chemical properties of aldehydes are:
Aldehydes are oxidized when they react with Tollens, Benedict and Fehling reagents. to form the corresponding carboxylic acid. The acid formed will have the same number of carbons in the carbon chain as the aldehyde from which it was formed. For example:
- Oxidation with Tollens reagent (ammoniacal silver complex in basic solution, [Ag (NH3)2]+). This reaction produces the corresponding acid and metallic silver.

- Oxidation with Benedict and Fehling reagent (alkaline solutions of copper (II) sulfate (CuSO4) with different compositions). This reaction produces the corresponding acid and cuprous oxide (Cu2EITHER).

They undergo nucleophilic addition reactions, where nucleophiles are added to the carbonyl group of aldehydes. Some examples are:
- addition reactions alcohols to the carbonyl group of aldehydes to form acetals and hemiacetals.
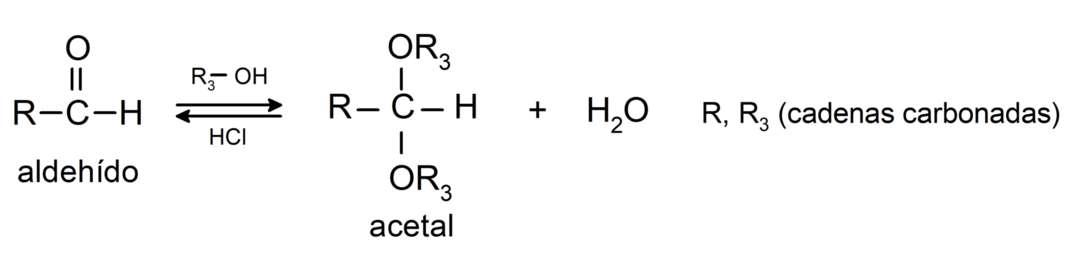
- addition reactions amines primary to the carbonyl group.

- Hydrocyanic acid (HCN) addition reactions, where cyanohydrins or cyanohydrins are formed.

They undergo aldol condensation reactions. In this type of reaction, two aldehydes condense to form an aldol. They are reactions that occur with sodium hydroxide (NaOH). For example:
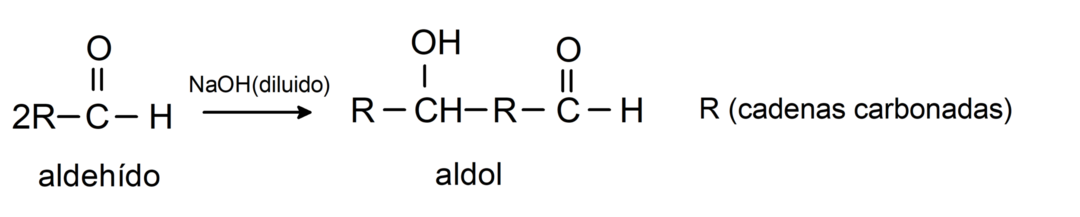
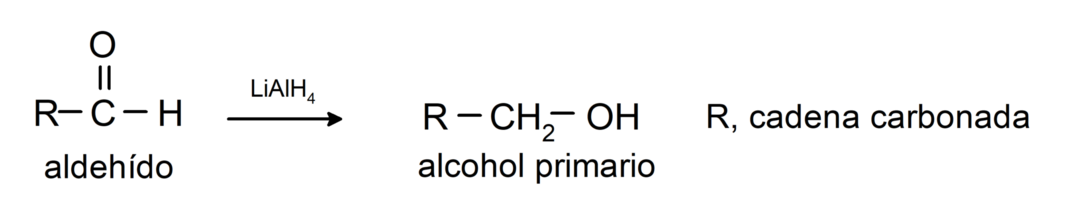
They undergo reduction reactions to primary alcohols. In the presence of sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) or by catalytic hydrogenation, they are reduced to primary alcohols. For example:
Examples of aldehydes
- methanal (formaldehyde)
- ethanal (acetaldehyde)
- propanal (propaldehyde)
- butanal
- pentanal
- vanillin
- cinnamaldehyde
- propenal
- benzaldehyde
- hexanal
- 3-bromocyclopentanecarbaldehyde
- cyclohexanecarbaldehyde
- 4,4-dimethylpentanal
- 2-hydroxy-butanal
- 2-hydroxy-2-methyl-butanal
- 2,3-dimethylpentanal
- pentanedial
- cyclopentanecarbaldehyde
- isobutanal
- 2-chloro-butanal

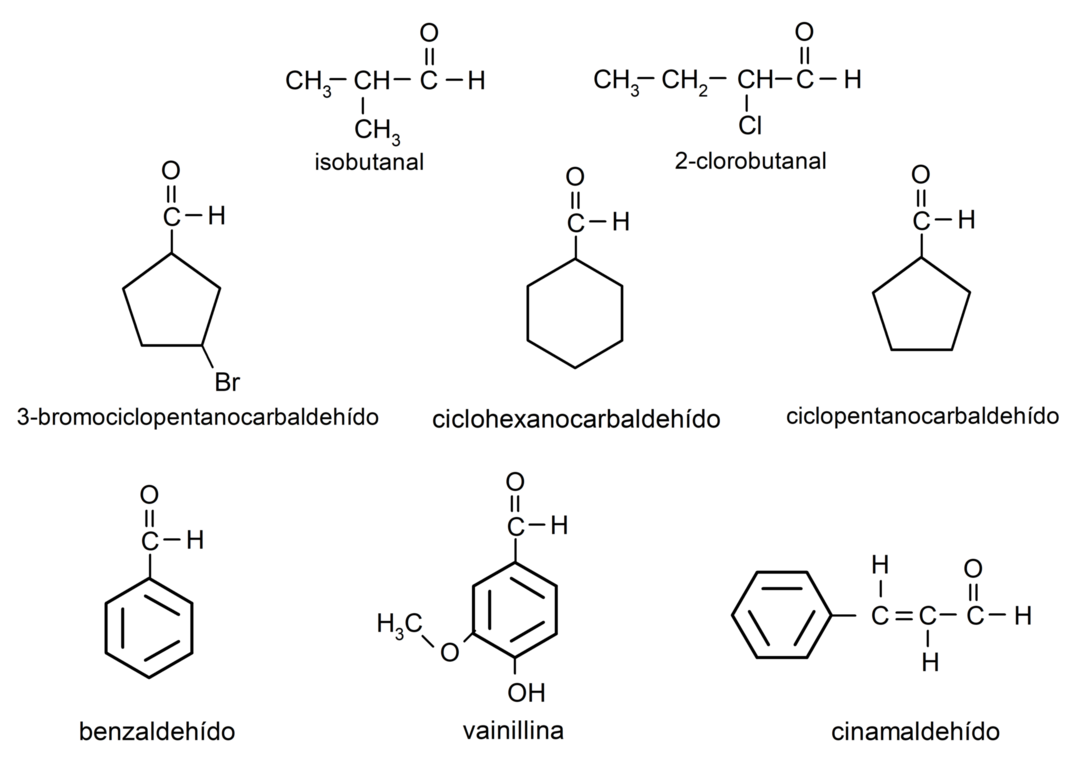
Aldehydes in nature
Some aldehydes present in nature are:
- Benzaldehyde, a component of almonds.
- Cinnamaldehyde, the component that gives the cinnamon essence its smell.
- Vanillin, the component that gives vanilla its flavor.
On the other hand, one of the forms of glucose, the open form, has an aldehyde functional group.
Acetaldehyde, which is formed as an intermediate in the metabolization of alcohol, is believed to cause hangover symptoms when intoxicated by alcohol.
Uses of aldehydes
Some of the main uses of aldehydes are:
- They are used in the production of solvents, paints, cosmetic products and essences.
- They are used in the manufacture of resins. Bakelite is made with formaldehyde and is a resin that works very well as an electrical insulator.
- They have been used as sedatives. Paraldehyde has been used as a sedative and hypnotic, although it is now out of use due to its unpleasant odor.
- They are used as preservatives for biological samples and corpses. Formaldehyde is widely used in this sense.
- They are used as food flavorings. An example is vanillin, which is used to give desserts a vanilla aroma.
- They are used as disinfectant agents.
Dangers of aldehydes
Several aldehydes have been considered carcinogenic, for example, formaldehyde has been declared a carcinogenic compound according to the WHO (World Health Organization).
Exposure and contact with many aldehydes causes irritation to the skin, eyes and respiratory tract. On the other hand, it causes contact dermatitis and liver diseases.
References
- Solomons, T.W. Graham and María Cristina Sangines Franchini (1985). “organic chemistry” Mexico, D.F.: Limusa.
- Whitten, K. W., Gailey, K. D., Davis, R. E., de Sandoval, M. T. TO. O., & Muradás, R. M. g. (1992). “General chemistry" (pp. 108-117). McGraw-Hill.
- Arteaga, P. M. (2017). “Ketones and aldehydes” Con-Science Scientific Bulletin of the Preparatory School No. 3, 4(8).
Follow with:
- Sugars
- Hydracids
- Ethyl alcohol
- Organic and inorganic compounds


