Examples of Endothermic Reactions
Examples / / November 09, 2023
A endothermic reaction It is a reaction that absorbs energy from the medium in the form of heat. In these reactions, for the reactants to transform into products, it is necessary for them to absorb heat, which causes the products to have greater energy than the reactants that gave rise to them. Some examples of endothermic reactions are: photosynthesis and water electrolysis.
- See also: Chemical reactions
Examples of endothermic reactions in everyday life
Some of the main endothermic reactions are:
Ozone production in the atmosphere. Ozone is produced in the atmosphere when molecular oxygen (O2) absorbs ultraviolet radiation and breaks down. Then an oxygen atom (O) can interact with another oxygen molecule (O2) and form ozone (O3).

Water electrolysis. It is the process by which it is applied electric power to water to separate it into its two components, hydrogen (H) and oxygen (O).
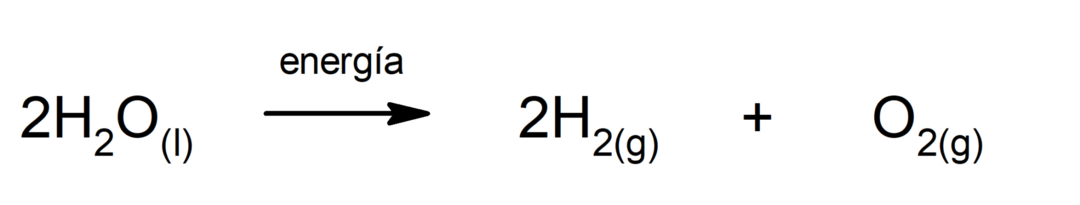
Photosynthesis. It is the chemical reaction by which, absorbing solar energy, carbon dioxide (CO2) is transformed into glucose. This reaction constitutes the main route of nutrition for plants.
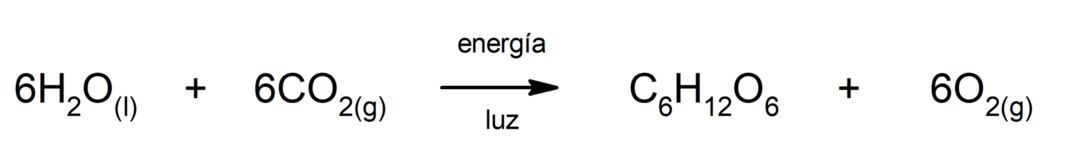
Iron(II) sulfide production. For the reaction between sulfur and iron to occur, it is necessary to provide energy in the form of heat.
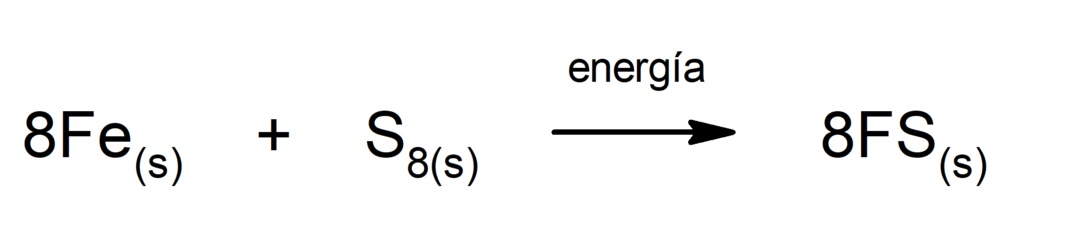
Decomposition of carbon dioxide (CO2). The decomposition of CO2 At high temperatures it produces carbon monoxide (CO) and oxygen (O2).
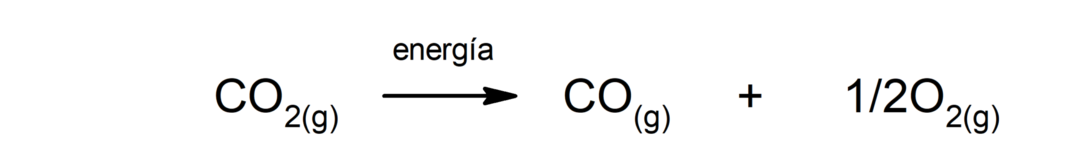
Decomposition of calcium carbonate (CaCO3). Calcium carbonate decomposes with absorption of heat to produce calcium oxide (CaO) and carbon dioxide (CO2).
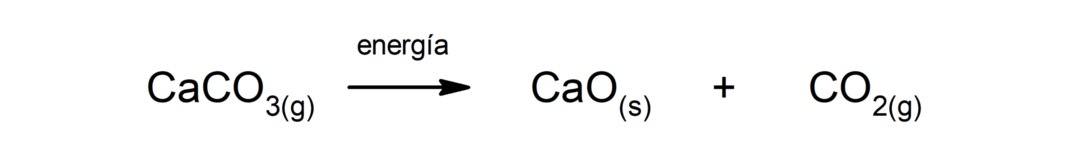
Difference between endothermic and exothermic reaction
The main difference between endothermic and exothermic reactions is that endothermic reactions absorb heat, while exothermic reactions release heat.
Furthermore, endothermic reactions are characterized by an increase in enthalpy, while exothermic reactions are associated with a decrease in enthalpy.
Enthalpy is a thermodynamic quantity that defines the flow of thermal energy at constant pressure during a chemical reaction. It is represented by the letter H, and its variation is one of the main indicators to define whether a chemical reaction is endothermic or exothermic.
- If a chemical reaction has an enthalpy change greater than zero (ΔH > 0) is endothermic.
- If a chemical reaction has an enthalpy change less than zero (ΔH < 0) is exothermic.
Follow with:
- Physicochemical phenomena
- Chemistry in everyday life
- Chemistry in everyday life
- Organic and inorganic chemistry
References
- Soto-Córdoba, S. (2016). Endothermic Reactions. Tech Repository Technological Institute of Costa Rica.
- Corominas, J. (2017). Chemical reactions of everyday life. Alembic, (90), 8-26.
- Sánchez, M. T. M., & Sánchez, M. M. (2002). Experimental study of endothermic reactions for ESO students. Annals of Chemistry of the RSEQ, (4), 36-39.



