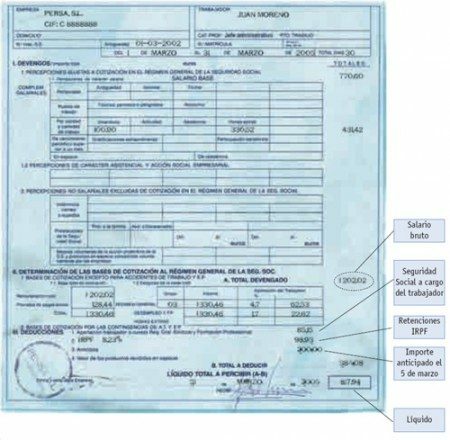
Chemistry in Everyday Life
Chemistry / / July 04, 2021
Although it seems that chemicals are only present in laboratory work or in industry, it is a reality that are present in all our daily activities, fulfilling functions and satisfying needs on a day-to-day basis. Chemistry is present in everyday life from the simplest compounds to the complex molecules of biochemistry.
Chemistry in our daily activities
In our daily activities, there are many chemicals present, such as EDTA (Ethylenediaminetetraacetic Acid) contained in the shampoo that we use to wash our hair. Its utility is to capture the Calcium and Magnesium Carbonates that form the hardness of water, so that the hair is smooth and silky.
The toilet soap contains Sodium Stearate or Potassium Stearate as the main ingredient, product of the saponification of fats through a Sodium or Potassium Hydroxide.
The hair styling gel contains Triethanolamine, which is a trialcohol that functions as an emulsifier and surfactant, since the other ingredients do not naturally hold together.
Women who need to remove their nails use a solvent whose base component is
Diethyl ketone, commercially called Acetone.Toothpaste contains as a key ingredient the Sodium fluoride, in charge of cleaning and whitening teeth. The industry complements it with flavorings to make it more attractive to customers.
The pencils we write with are made from a wooden shell, and the active ingredient is an allotropic form of Carbon, which is Graphite.
The sheets on which you write and print are made of Cellulose, the same material that gives structure to plants. Cellulose is a long chain polysaccharide, although not as long as that of starch.
Tools used for mechanical fixes, such as wrenches and pliers, are made from a combination of metals Chromium and Vanadium.
Chemistry in the home
In the refrigerator, the agent that is responsible for cooling the food is a gas that circulates through an internal network. Gas at an industrial level can be Ammonia, but at home it is a Chlorine Fluoro Carbon, such as Refrigerant R-134, which is DiCloroDiFluoroethane.
To disinfect food, drops of Colloidal Silver, which come in a dropper presentation. It is generally used in fresh vegetables such as lettuce. It is left to rest for a moment for the disinfection to take effect, and then the vegetable is washed again.
When cooking, vegetable oil is used whose basic composition consists of Unsaturated carboxylic acids, like Oleic Acid, Linoleic Acid and Linolenic Acid.
Heating in the stove is achieved with combustion, a chemical reaction in which a fuel degrades releasing a large amount of heat. Domestic fuel is usually in a gaseous state, such as Natural Gas or Methane, and LP Gas (Liquefied Petroleum gas), made up of Propane and Butane, hydrocarbons that occupy the third and fourth place among alkanes.
To intensify the flavor of the food, Table Salt is added, whose chemical representative is the Sodium chloride. If the food is sweet, a sweetener is added, such as Sugar or Sucrose, or some alternative like Sucralose, 600 times sweeter than Sucrose.
The utensils that are used at mealtime are made with a zinc coating, the placement of which occurs in an electrolytic procedure called Galvanized.
To clean the fat embedded in a stove, the base is used Sodium hydroxide in solution, which is sufficiently diluted so that it does not cause severe damage to human skin.
To clean the toilet, use a diluted solution of Hydrochloric acid, whose trade name is Muriatic Acid. Leaving it to act for a moment on the areas to be cleaned, it is removed with a wash at the end.

For cleaning floors, mixtures of Sodium lauryl ether sulfate, with fixative, flavoring and surfactants. They leave a shiny, dirt-free appearance.
If you have a medicine cabinet at home, you will have a disinfectant based on Hydrogen peroxide (Hydrogen peroxide), or a bottle of ethyl alcohol, and the essential drugs for mild ailments, such as Acetylsalicylic acid for headaches, Paracetamol for fevers, Omeprazole Y Milk of magnesia (Magnesium Hydroxide), both for heartburn.
Chemistry in the human body and food
The human body is the sample of the presence of infinity of chemical compounds contained in the same organism. For starters, between 70 and 75% of your weight is Water, from there we go through the skeleton, made of calcium and phosphorus mainly, the muscles, which are a mixture of amino acids that together create protein chains, tissues, which are natural polymers and that together with a layer of lipids or fats they help us conserve heat and energy. Hair and nails are made of Keratin, and the skin color is attributed to a compound called Melanin.
In addition to the compounds that form it structurally, there are also those that fulfill specific functions within organs, such as insulin, produced in the Pancreas to regulate the amount of Glucose circulating in the body, or the testosterone and progesterone, male and female hormones respectively. The Hemoglobin, for example, is responsible for transporting oxygen from the lungs throughout the body in the bloodstream. There are also enzymes that accelerate the assimilation of nutrients, such as amylase, which serves to better digest glycogen and starch.
At the time of feeding, the intention is to provide the body with the nutrients it needs. The most important are called Macronutrients, because they are required in greater amounts than the others. Among them are the protein, the carbohydrates and the lipids. Everything we eat contains them, to a greater or lesser extent, and it is up to us to eat a diet that balances them as best as possible, because excess of any is bad.
20 examples of Chemistry in everyday life
- The combustion of natural gas in the stove
- The chemical attack of caustic soda on the fat stuck in the stove
- The chemical attack of muriatic acid on toilet dirt
- Electrolyte solution from car battery
- Adding salt to food to make the flavor stronger
- The action of Sodium Fluoride in tooth cleaning
- The beginning of digestion with gastric acids
- The action of soap salts to remove dirt from the body.
- The gas that circulates through the internal network of the refrigerator
- Sugar dissolving in coffee cup
- Alcohol that no longer evaporates when the drink is cooled with ice
- Leaving the dough when the cookies are baked
- The Carbon Dioxide that escapes little by little from the carbonated drink
- The Acetone with which women remove their nails
- The hand cream contains glycerin, which moisturizes the skin
- Antibacterial gel and hair gel contain the emulsifier Triethanolamine
- Chlorine used to disinfect water, used in minimal quantities
- Colloidal silver used to disinfect food
- Sodium Bicarbonate used to gargle during a sore throat
- Magnesium Hydroxide in Milk of Magnesia, which regulates heartburn