Subatomic Particles Example
Chemistry / / July 04, 2021
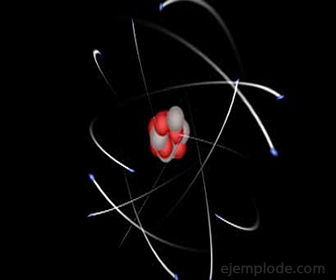
The Subatomic particles They are tiny units that make up the Atom. The most important are three: the Proton and the Neutron forming the Atomic Nucleus, and the Electron, orbiting around the latter.
Matter, everything that encompasses a site in space, is made up of fundamental units called Atoms. The number of different atoms that exist is the number of Chemical elements on the Periodic Table.
Different combinations of Atoms constitute all that we know; These combinations are the object of study of both, the Inorganic chemistry and the Organic chemistry.
But it is also explored into the interior of the atoms, which coincide in having a basic structure, which is made up of lower particles called the Atomic Nucleus and Electrons.
The Atomic nucleus It is made up of two different types of particles: Protons and Neutrons.
The Protons carry a positive electric charge (+) and the Neutrons carry no charge. The Electrons carrying a negative charge (-) They interact with the charge of the Protons, and a phenomenon of attraction is generated that keeps the atom in a certain state of Energy.
An atom is said to be Stable when positive and negative charges completely cancel each other out.

Electron
Air at ordinary pressure conducts electrical current very poorly. But rarefied air, as it exists in a vacuum discharge tube, conducts the current in the form of a beam of particles called Cathode Rays. In 1879, Sir William Crookes proved that particles carried an electric charge.
In 1895, Jean Perrin was able to verify that the charge is negative; and the particles were given the name Electrons. The same year, studying the deflection of rays in an electric field, Sir J. J. Thompson determined the value of the Specific Charge, which is the ratio between the charge of the Electron (e) and the mass (m) of the Electron.
From the value 1.7592 * 108 Coulombs / gram of "e / m" and the value of "e" (1.602 * 10-19 Coulombs), first determined by R. TO. Millikan in 1917, the mass of the electron was calculated, which is 1/1838 of the mass of the Hydrogen atom.
Electron Charge = 1.602 * 10-19 Coulombs
Electron mass = 1/1838 of the mass of the Hydrogen atom
The first determinations of the charge of the electron were made by Townsend (1897), J. J. Thomson and by H. TO. Wilson (1903), the latter using the camera of C. T. R. Wilson (1897) to produce mists, a device widely used in the investigation of atomic structure.
Electrons are found in the outer part of the Atom, describing a movement around the Nucleus, as well as the planets around the Sun. The number of electrons around the Nucleus is what tells which Chemical Element it is.
For example, if there is only one electron in the atom, the Element is Hydrogen. If there are 23 electrons, it is Sodium. If there are 80 electrons, the Element is Mercury.
Proton
When an electric current is passed through a vacuum tube in which a perforated disk acts as a Cathode (negative electrode), the Cathode Rays (electrons) are directed towards the Anode (electrode positive); but on the other side of the cathode, positively charged particles appear that can be deflected by a powerful magnetic field.
The charge of these particles, although positive, is always equal to or a multiple of that of the electron. The mass of a positively charged particle varies according to the nature of the gas enclosed in the tube; in general it is equal to that of the gas atom. The bundles of these particles are called Positive Rays.
If the tube contains Hydrogen, each positive particle has approximately the mass of a Hydrogen atom, and its charge is equal in magnitude to that of the electron. The hydrogen atom is the lightest and simplest of all atoms, and the positive ray particles obtained from it are the lightest and simplest of all positive particles.
Proton Charge = 1.602 * 10-19 Coulombs
Mass of Proton = Mass of Hydrogen Atom
Rutherford found that this same positive particle is frequently produced by bombarding different elements with rays emitted by Radium. He called this simpler positive particle Proton, and drew the conclusion that it is a constituent of the Atom.
Neutrons
Today it is commonly accepted that an atom is composed of a small nucleus with positive electric charges equal in number to the Atomic Number (number of electrons orbiting around the nucleus) in the center or very close to it, of the space available for the entire Atom and of negative electrons in the outer part of said space.
The number of electrons coincides with the number of positive charges in the Nucleus. With the exception of the Hydrogen Atom, the mass of the atom is explained by the fact that the Nucleus contains not only Protons, but a certain number of neutral particles, which They were first considered as neutralized protons (each combined with an electron), but today they have been recognized as fundamental units of matter with mass, named Neutrons.
Other Subatomic Particles
In addition to electrons, protons and neutrons, other particles considered also as constituents of atoms are currently known: they are the Positron, the Meson or Mesotrón and the Neutrino.
The Positrons were discovered by Carl Anderson (1932) in the interaction of cosmic rays (radiation that reaches Earth from Space) with matter, and in certain processes of radioactivity artificial. Positrons are identical to electrons, only their charge is positive instead of negative. Their existence as free particles is extremely small, being less than a millionth of a second.
The Mesons They were also discovered by Carl Anderson in collaboration with Seth Neddermeyer (1936) by the action of Cosmic Rays with matter. They have a mass, it appears to be non-constant and approximately equal to one-tenth that of the Proton, and a positive or negative electrical charge. They have a very short life and are supposed to decompose into Neutrinos plus Electrons or Positrons. The attempt to artificially obtain mesons in the laboratory, with the use of ion accelerators and electrons (cyclotron, betatron, synchrotron, etc.) that supply these enormous energies, has been achieved in 1948.
The Neutrinos They are particles with mass equal to that of electrons and positrons, but without electric charge. Its existence was supposed by Fermi in 1925 to explain certain energetic calculations in the emission of Beta Particles by radioactive substances. Although new experiments can be perfectly explained by the existence of neutrinos, conclusive proof of it has not been found.
Examples of Subatomic Particles
Proton
Neutron
Electron
Positron
Meson or Mesotrón
Neutrino
Leptons
Quarks
Gluons
Photons
Hadrons
Graviton (theoretical particle)


