Covalent Bond Example
Chemistry / / July 04, 2021
The Covalent bond is the one in which two atoms unite by sharing their electrons, to go completing its Rules of the Octet.
History of the Covalent Bond
It was in the early 20th century that chemists began to understand how and why molecules were formed. The first important advance came with the proposition of Gilbert lewis about what the formation of a chemical bond implies that atoms share electrons. Lewis described the formation of a chemical bond in Hydrogen as:
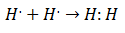
This type of electron pairing is an example of a Covalent Bond, a bond in which two electrons are shared by two atoms. The Covalent Compounds They are that contain only covalent bonds.
Electrons in the Covalent Bond
For simplicity, the shared electron pair is often represented as single line connecting the symbols of the elements. Thus, the covalent bond of the Hydrogen molecule is written as H-H.
In the covalent bond, each electron of the shared pair is attracted to the nuclei of both atoms. This attraction holds the two atoms in the H molecule together.
2 and it is responsible for the formation of covalent bonds in other molecules.In the covalent bonds between atoms of several electrons only valence electrons participate, which are the outermost, in the shallowest orbital. Between one and three of them will participate in the union.
The other electrons, which do not participate in the bond, are called Non-Bonding Electrons, or if we organize them in pairs, Free Pairs. That is, pairs of Valencia Electrons that do not participate in Covalent Bond Formation.
Covalent Bond Representation
The structures with which covalent compounds are represented, such as H2 and F2 are known as Lewis structures. A Lewis structure is a representation of a covalent bond, where the pair of shared electrons indicated by lines or as pairs of points between two atoms, and the unshared free pairs are indicated as pairs of points on the individual atoms. In a Lewis structure, only the valence electrons are shown, and not the internal ones.
Considering the Lewis structure for the Water molecule H2Or, first all the valence electrons of the hydrogen and oxygen atoms are marked with dots.
In a second case, the link is marked with a line. And the free pairs, which will exist only in Oxygen, with points.
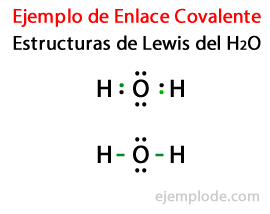
The Rule of the Octet
The formation of these molecules, like those of Water H2Or, illustrate the call Octet rule, proposed by Lewis: An atom other than Hydrogen tends to form bonds until it surrounds itself with eight valence electronsThat is, a covalent bond forms when there are not enough electrons for each individual atom to complete its octet.
By sharing electrons in a covalent bond, each atom completes its octet. For Hydrogen, the requirement is that you obtain the electronic configuration of Helium, which is to have a total of two electrons.
The octet rule works mainly for the elements of the second period or row of the periodic table. These elements have sublevels in which there can be a total of eight electrons.
When an atom of these elements forms a Covalent Compound, it obtains the electronic configuration of the Neon Noble Gas, sharing electrons with other atoms in the same compound.
Types of Covalent Bonds
Atoms can form different types of Covalent Bonds: Singles, Doubles or Triples.
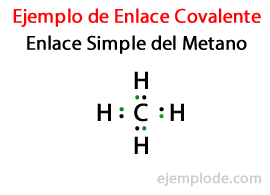
In a Simple Link, two atoms are joined by means of A Pair of Electrons. They occur in the vast majority of Covalent compounds, and it is the most basic form of this bond.
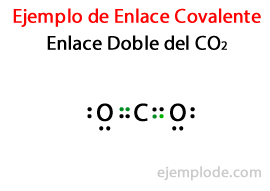
In many compounds, Double Links, that is, when two atoms share Two Pairs of Electrons. If two atoms share Two Pairs of Electrons, the Covalent bond is called a Double Bond. These bonds are found in molecules like Carbon Dioxide (CO2) and Ethylene (C2H4).
A Triple Link arises when two atoms share Three Pairs of Electrons, as in the Nitrogen N molecule2, the Acetylene C molecule2H2.

Multiple bonds are shorter than single covalent bonds. The Link length is defined as the distance between the nucleus of two joined atoms by a covalent bond in a molecule.
Differences between Covalent and Ionic Compounds
Ionic and covalent compounds present marked differences in their general physical properties, due to the fact that their bonds are of different nature.
In the Covalent Compounds exist two types of attractive forces; one of them is the one that holds the atoms of a molecule together. A quantitative measure of this attraction is the binding energy. The other force of attraction operates between the entire molecules, and is called Intermolecular force. Since Intermolecular Forces are usually weaker than the forces that hold the atoms of a molecule together, the molecules of a covalent compound bond with less force.
In consecuense, covalent compounds are almost always low-melting gases, liquids, or solidsn. On the other hand, the electrostatic forces that hold the ions together in an ionic compound they are usually very strong, so that ionic compounds are solid at room temperature and have high melting points. Many ionic compounds are soluble in water, and their aqueous solutions conduct electricity because these compounds are strong electrolytes.
Most of the covalent compounds are insoluble in water, and if they dissolve, its aqueous solutions as usual they do not conduct electricity because these compounds are nonelectrolytes. Molten ionic compounds conduct electricity because they contain cations and anions that move freely; liquid or molten covalent compounds do not conduct electricity because there are no ions present.
Examples of Covalently Bonded Compounds
- Acetylene C2H2
- Methane CH4
- Ethane C2H6
- Propane C3H8
- Butane C4H10
- Benzene C6H6
- Toluene C7H8
- Methyl Alcohol CH3Oh
- Ethyl Alcohol C2H5Oh
- Propyl Alcohol C3H7Oh
- Methyl Ether CH3OCH3
- Methyl Ethyl Ether C2H5OCH3
- Ethyl Ether C2H5OC2H5
- Formic Acid HCOOH
- Acetic Acid CH3COOH
- Propionic Acid C2H5COOH
- Butyric Acid C3H7COOH
- Carbon Dioxide CO2
- Carbon Monoxide CO
- Molecular nitrogen N2
- Molecular hydrogen H2

