Example of Nomenclature of Organic Compounds
Chemistry / / July 04, 2021
Organic compounds are molecules whose characteristic is that they are formed by a base of carbon molecules and hydrogen, also known as the skeleton, and combined with other elements, mainly oxygen, nitrogen, and sulfur.
The IUPAC (International Union of Pure and Applied Chemistry, International Union of Pure and Applied Chemistry) has established general rules for the classification and nomenclature of organic molecules, of which we explain the most important.
Hydrocarbons
Hydrocarbons are the simplest organic molecules, made up of a carbon skeleton and hydrogen atoms. There are three types of hydrocarbons:
Alkanes
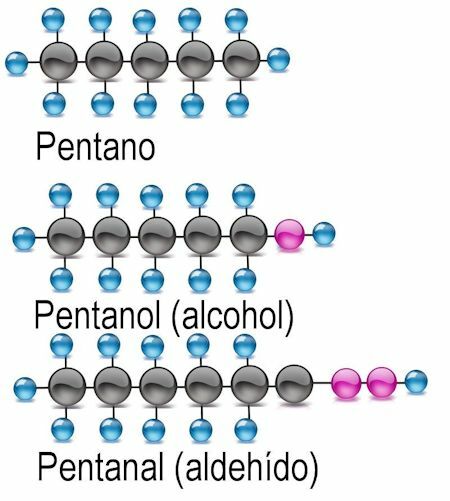
They are the simplest, formed by carbon atoms joined by simple covalent bonds to the atoms of hydrogen, with the general formulation H = 2n + 2, that is, the hydrogen atoms are twice the number of hydrogen atoms. carbon, plus 2. Its nomenclature is formed with the numerical prefixes met- for 1 carbon atom, et- for 2, prop- for 3, and but- for 4; from 5 carbon atoms the common prefixes pent-, hex-, hept-, oct-, etc. are used. To all alkanes the ending -ano is added.
Example: CH4: methane; C2H6: ethane; C4H10: butane; C6H14: hexane
Alkenes
They are formed by a carbon skeleton in which there is a double bond between the carbon atoms. Its general formula is H = 2n, that is, its hydrogen atoms are twice the number of carbon atoms. Like alkanes, they use prefixes that indicate their number of carbon atoms, and in this case the ending -eno is added. In addition, in molecules with more than three carbon atoms, the carbon where the double bond is found, starting to count from the carbon closest to is.
Examples: C2H4: ethene; C3H6: propene; C4H8: butene, 2-butene; C6H12: Hexene, 2-hexene (double bond at atom 2), 3-hexene (double bond at atom 3).
Alkynes
Alkynes contain a triple bond at their carbon atoms. Its general formula is H = 2n-2, indicating that the molecule will contain two hydrogen atoms less than twice the number of carbon atoms. To the prefix that indicates the number of carbons, the ending -ino is added. As in the case of alkenes, the carbon containing the triple bond is mentioned, counted from the extreme closest to it.
Examples: C2H2: ethyne; C3H4: tip; C4H6: butyne, 2-butyne; C6H12: Hexino, 2-hexene (triple bond at atom 2), 3-hexyne (triple bond at atom 3).
Functional groups
Functional groups are combinations of atoms that function as an ion and replace a hydrogen atom in the formula of an alkane.
Radicals
The alkane without the hydrogen atom is called Radical, and to identify it the ending -ilo is added:
Examples CH4: methane - CH3+ methyl; C2H6: ethane - C2H5+ ethyl; C4H10: butane - C4H9+ butyl; C6H14: hexane - C6H13+ hexyl.
Combination of hydrocarbons
One of the cases that can occur is that two or more hydrocarbons are combined in a molecule. The hydrocarbons with which it is combined are called arborescences. In these cases, the base hydrocarbon will be the one with the longest chain, and the number of carbon where the arborescences are found, followed by the name of the radical with the ending -il, and then the name of the hydrocarbon base. The number will be mentioned as many times as the trees attached to it. In the case that the arborescences are of the same radical in different carbon atoms, the numbers separated by commas will be mentioned, followed by a hyphen, the name of the radical, then the numbers of the atoms where the other radical is attached, if any, a dash, the name of the radical and the name of the hydrocarbon base. In case there are two radicals attached to the same carbon atom, the number will be mentioned twice. If they are different radicals, then it will be mentioned once before the name of each radical; if the radicals are equal, the number will be mentioned twice. We will start by mentioning the simplest arborescences first (those with the least amount of carbons) and then those with the highest amount.
Example: A pentane molecule, with two ethane radicals, attached at carbon 2 and 3:
2,3-ethyl pentane. An octane molecule, a propane radical at carbon 6, methane radicals at carbons 4 and 5, and ethane radicals, bonded at carbons 2, 3, and 4: 4,5-methyl-2,3,4-ethyl-6- propyl-octane.
Alcohol functional group
The simplest of the functional groups is alcohol, in which a hydrogen atom is replaced by a hydroxyl group (OH). In these compounds the ending -anol is added to the name of the radical. Where appropriate, the carbon atom where the functional group is found should be mentioned:
Examples CH3OH: methanol: C2H5OH ethanol; C4H9OH butanol or 2-butanol; C6H13OH hexanol, 2-hexanol (functional group at atom 2), 3-hexanol (functional group at atom 3).
Aldehyde functional group
In aldehydes, the hydrogen atom is exchanged for the functional group -CHO. To identify them, the ending -anal is added, and the carbon atom where the functional group is found is also mentioned:
Examples CH3COH: methanal: C2H5Ethanal COH; C4H9COH butanal or 2-butanal; C6H13COH hexanal, 2-hexanal (functional group at atom 2), 3-hexanal (functional group at atom 3).
Acid functional group
In organic acids, the hydrogen atom is exchanged for the functional group -COOH. To identify them, the word acid is mentioned and the ending -anoic is added, and the carbon atom where the functional group is found is also mentioned:
Examples CH3COOH: methanoic acid: C2H5COOH ethanoic acid; C4H9COOH butanoic acid or 2-butanoic acid; C6H13COH hexanoic acid, 2-hexanoic acid (functional group at atom 2), 3-hexanoic acid (functional group at atom 3).
