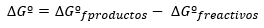Example of Endothermic Reactions
Chemistry / / July 04, 2021
Thermochemistry is the part of chemistry that is responsible for the study of the relationship between chemical reactions and changes in temperature during reactions. When two substances react, the reaction can be endothermic or exothermic.
Exothermic reactions are those that produce heat.
Endothermic reactions are those in which while two or more substances are reacting, they absorb external energy in the form of heat. Once the reaction is finished, the resulting product is called an exothermic body, because when it decomposes, it produces heat.
The main characteristics of endothermic reactions are the following:
- To carry out the reaction, it is required to add heat to the reactants.
- The heat input must be constant so that the reaction is completely finished, if it is interrupted, the reaction does not finish.
- The resulting substance (exothermic body), are chemically unstable compounds, which decompose easily.
- When exothermic bodies decompose, they give off the heat that they absorbed during the endothermic reaction that formed it.
- When the decomposition reaction begins, the chain reaction continues until complete decomposition.
Example of endothermic reactions:
In the higher layers of the atmosphere, oxygen molecules (O2), are subjected to the action of the energy of ultraviolet rays, which provide thermal energy so that the oxygen molecules react with each other, producing ozone:
3O2 + heat -> 2O3
These ozone molecules are unstable, and once they stop receiving UV energy, they dissociate into oxygen molecules. This dissociation reaction releases the heat absorbed during ozone formation.
Another example of an endothermic reaction is nitrous oxide. Combining oxygen and nitrogen requires adding heat to the mixture.
2N2 + O2 + heat -> 2 N2OR
Nitrous oxide, once it begins its decomposition, gives off a large amount of energy, so it is used as an accessory to instantly increase the power of a car. However, if the amount of oxide supplied to the engine is excessive, the amount of heat produced can melt the pistons and damage the engine.