Magnetic Materials Example
Physics / / July 04, 2021
The Magnetic Materials are those who are capable of producing a Force Field that attracts metallic materials, Campo also called Magnetic Field.
Magnetism
The Magnetism is the capacity of a material of produce a magnetic field, which will be in charge of hauling the metals that are close to it.
It is possible that electric currents produce a magnetic field passing through a material, making it magnetic. This phenomenon is called Electromagnetism. In addition to this option, there are natural or synthetically created materials, which create a magnetic field.
The fields created by magnetic materials come from two atomic sources: the orbital angular moments Y spin of electrons, that being in continuous movement in the material, they experience forces before a magnetic field that is applied.
The magnetic characteristics of a material can change by mixing or alloying with other elements, where they are altered by the interactions between atoms.
For example, a non-magnetic material such as aluminum can behave as a magnetic material in materials such as the Alnico (Aluminum-Nickel-Cobalt) or Manganese-Aluminum-Carbon mixture.
Also, non-magnetic material can take on this characteristic through mechanical work or other source of stress that changes the geometry of the crystalline lattice that originally conforms it.

Magnetic Moments
All material is composed of atoms containing mobile electrons. A magnetic field applied on it always acts on the electrons considered individually. This gives rise to the effect called Diamagnetism. This is a well-known phenomenon, and it depends solely on the movement of electrons.
Electrons are going to have a Magnetic Moment, what is a work done by them to create a Magnetic Field. The Magnetic Moment can be Orbital, due to the movement of electrons around the core, or Intrinsic or spin, which is due to the spin of the electron itself.
At the level of the atom, the splicing of Magnetic Moments, contributed by electrons to the atom or molecule of which they are part, gives a resulting magnetic moment to the atom or molecule.
When there is a net atomic or molecular moment, the magnetic moments tend to align with the applied field (or with the fields created by neighboring magnetic moments), resulting in the effect of Paramagnetism.
At the same time, the thermal energy present everywhere tends to randomly orient the moments so that the relative intensity of all these effects will determine the behavior of the material. In a Non-Magnetized material the Magnetic Moments are randomly oriented.
Magnetic Permeability
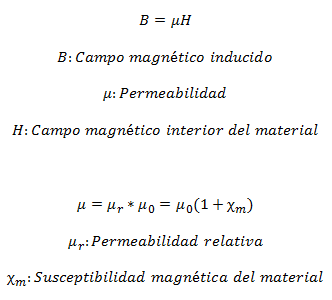
Magnetic materials are characterized by their Permeability µ, which is the relationship between magnetic induction field (the one that is contributed) and the magnetic field within the material:
Magnetic Behaviors
Materials that can be modified with a magnetic field can behave in various ways, including the main ones are Diamagnetism, Paramagnetism, Ferromagnetism, Antiferromagnetism, and Ferrimagnetism.
Diamagnetism
The Diamagnetism is an effect that is based on the interaction between the applied field and mobile electrons of the material.
Diamagnetic materials are weakly magnetize in the opposite direction that of the applied magnetic field. It turns out that a repulsive force appears on the body with respect to the applied field.
Examples of diamagnetic materials are copper and helium.

Paramagnetism
The materials Paramagnetic are characterized by atoms with a net magnetic moment, which are usually aligned parallel to an applied field. The properties of paramagnetism are as follows.
Paramagnetic materials are weakly magnetized in the same direction than the applied magnetic field. It turns out that an attractive force appears on the body with respect to the applied field.
The intensity of the response is very small, and the effects are practically impossible to detect except at extremely low temperatures or very strong applied fields.
Examples of paramagnetic materials are aluminum and sodium. Different variants of paramagnetism occur as a function of the crystalline structure of the material, which induces magnetic interactions between neighboring atoms.

Ferromagnetism
In the materials Ferromagnetic the individual magnetic moments of large groups of atoms or molecules they stay aligned with each other due to a strong coupling, even in the absence of an external field.
These groups are called Domains, and they act like a small permanent magnet. The domains are formed to minimize the magnetic energy between them.
In the absence of an applied field, the domains have their net magnetic moments randomly distributed. When an outer field is applied, the domains tend to align with the field. This alignment can remain in some cases of very strong coupling when the field is removed, creating a permanent magnet. Thermal agitation tends to misalign the domains.
Ferromagnetic materials are strongly magnetized in the same direction as the magnetic field applied. Thus, an attractive force appears on the body with respect to the applied field.
At normal temperature, thermal energy is generally not sufficient to demagnetize a magnetized material. However, above a certain temperature, called the Curie Temperature, the material becomes paramagnetic.
One way to demagnetize a ferromagnetic material is then heat it above this temperature.
Examples of ferromagnetic materials are iron, cobalt, nickel, and steels.

Antiferromagnetism
The materials Antiferromagnetic they have a natural state in which the atomic spins of adjacent atoms are opposite, so that the net magnetic moment is zero. This natural state makes it difficult for the material to magnetize.
Manganese Fluoride (MnF) is a simple example. Above a critical temperature, called the Neel temperature, an antiferromagnetic material becomes paramagnetic.
Another example of an antiferromagnetic material is chromium.

Ferrimagnetism
The materials Ferrimagnetic are similar to antiferromagnetics, except that the alternating atom species are different, such as for example, by the existence of two interlaced crystalline subnets and have magnetic moments different.
So there is a net magnetization, which can be very intense in cases. The Magnetite It has been known as a magnetic material since ancient times. It is one of the oxides of iron (Fe3OR4) and is of structure with cubic arrangement. Other examples of ferrimagnetic materials are ferrites.

The magnets
It is usually called Magnet to any object that produces an external magnetic field. A permanent magnet is a material that, when placed in a sufficiently strong magnetic field, not only produces its own or induced magnetic field, but also continues to produce induced field even after being removed from the applied field.
This property is not altered or weakened over time except when the magnet is subjected to temperature changes, demagnetizing fields, mechanical stresses, etc. The ability of the material to withstand without changes in its magnetic properties various types of environments and working conditions defines the types of applications in which it can be used.
Is named Soft Magnetic Material to the one that loses its magnetization when the external field that produced it is withdrawn. It is useful for transporting, concentrating or shaping magnetic fields.
The Hard Magnetic Materials they are those that sustain the magnetization even removing the applied field. They are used for the manufacture of permanent magnets.

Examples of Magnetic Materials
- Alnico Mix (Aluminum-Nickel-Cobalt)
- Manganese-Aluminum-Carbon Mixture
- Copper (Diamagnetic)
- Helium (Diamagnetic)
- Aluminum (Paramagnetic)
- Sodium (Paramagnetic)
- Iron (Ferromagnetic)
- Cobalt (Ferromagnetic)
- Nickel (Ferromagnetic)
- Steels (Ferromagnetic)
- Magnesium Fluoride MnF (Antiferromagnetic)
- Chromium (Antiferromagnetic)
- Magnetite Faith3OR4 (Ferrimagnetic)
- Ferrites (Ferrimagnetic)


