Concept in Definition ABC
Miscellanea / / July 04, 2021
By Cecilia Bembibre, in May. 2010
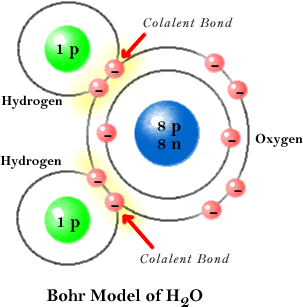 The word covalent is normally used to designate a type of bond that occurs between the electrons of different atoms. The covalent bond represents the sharing of (negative) electrons at a level that, however, is not enough to be able to speak of exchange of electrons between both atoms. These bonds between electrons enter into the sphere of chemical science.
The word covalent is normally used to designate a type of bond that occurs between the electrons of different atoms. The covalent bond represents the sharing of (negative) electrons at a level that, however, is not enough to be able to speak of exchange of electrons between both atoms. These bonds between electrons enter into the sphere of chemical science.
The covalent bond can be described, in other words, as the bond that is established between the electrons of different atoms and that generates the phenomenon of attraction-repulsion that occurs between them. This phenomenon (or covalent bond) is what maintains the stability between those atoms thus united through their electrons.
I know esteem that the term "covalent bond" began to be used in the early 20th century, more specifically in 1919, by Irving Langmuir. This scientist used the notion of covalent to designate those pairs of electrons shared by an atom with its neighboring atoms. The bonding of electrons between atoms can be simple (when one is shared), double or triple and so on form more or less complex substances according to the number of electrons and atoms that are related each.
Covalent bonds can give rise to two types of substances or main materials: those that are soft when in solid state, are insulators of the electric power, can be found in all three states (liquid, gaseous and solid) and have low boiling and melting ranges in comparison with other substances. These substances are called "molecular covalent substances". The second group is made up of substances that are only solid, are not soluble in any liquid or substanceThey have high melting and boiling temperatures and are insulating as well. We know them as network substances. Furthermore, these network substances are always very harsh.
Themes in Covalent