Definition of reaction rate
Miscellanea / / July 04, 2021
By Florencia Ucha, on Feb. 2014
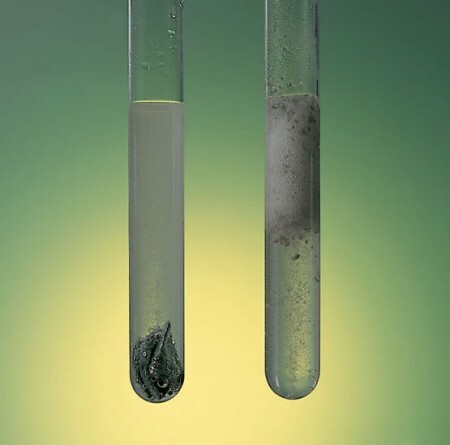 The concept of velocity reaction designates the amount of substance that is converted in a given reaction, per unit volume and time. Thus, the reaction of a material such as iron will be much slower and will take years to comparison with the combustion of butane gas, at the behest of a fire, which will occur in just a few seconds.
The concept of velocity reaction designates the amount of substance that is converted in a given reaction, per unit volume and time. Thus, the reaction of a material such as iron will be much slower and will take years to comparison with the combustion of butane gas, at the behest of a fire, which will occur in just a few seconds.
Meanwhile, it will be the chemical kinetics, that area within the physical chemistry the one in charge of studying the speed of a reaction and as certain variable conditions modify the speed of reaction of a material or substance, and also the molecular events that take place in the general reaction. Meanwhile, it will be the chemical dynamics the one that deals with studying the origin of the speeds of the various types of reactions.
It should be noted that fields such as those of the chemical engineering, environmental engineering and enzymology apply chemical kinetics in their processes.
There are several factors that influence the speed of the reaction and that it is necessary to list to know how they affect it ...
Nature of the reaction is decisive since there are some reactions that by their nature can be faster than others and vice versa. The amount of species reacted, the physical state of the particles and the complexity the reaction, are some questions that mark the way in this regard.
On the other hand, the higher the concentration the faster the reaction rate.
The pressure, for its part, also affects the reaction speed. Thus, the speed of gaseous reactions increases markedly with pressure, which is practically the same as increasing the concentration of the gas.
The order The reaction rate also exerts its influence since the order controls how the concentration affects the rate of the reaction in question.
And finally the temperaturehas its importance because when carrying out a reaction at a very high temperature it will provide greater Energy to the system and therefore the speed of the reaction will increase causing greater particle collisions.
Topics in Reaction Speed