20 Examples of Chemical Bases
Miscellanea / / July 04, 2021
Is named chemical base to all substances that, when dissolved in water, liberate hydroxyl ions (OH–). For example: calcium hydroxide, copper hydroxide, zinc hydroxide.
Chemical bases are also known as alkali, because by dissociating and releasing the hydroxyl groups (OH–), the pH of the solutions increases, that is, the solution becomes alkaline. This is contrary to what happens when a acid, because in that case the pH decreases and the solution becomes acidic.
The bases have a bitter taste characteristic. After his dissolution, the resulting solutions conduct the electric current (due to the presence of ions) and are usually caustic and irritating to the skin and other human and animal tissues.
The bases neutralize acids, often forming you go out. Alkaline solutions tend to feel slippery or soapy; This happens because they immediately produce the saponification of the fats present on the surface of the skin.
In the case of hydroxides their solubility depends on metal: those of group (I) are the most soluble in water, on the other hand, the hydroxides of the elements with oxidation degree (II) are less soluble and oxidation degree (III) or (IV) are almost insoluble. Amines and bases of
nucleic acids they are the most widespread bases among the organic ones.Theories that define the bases
There are different theories to define chemical bases:
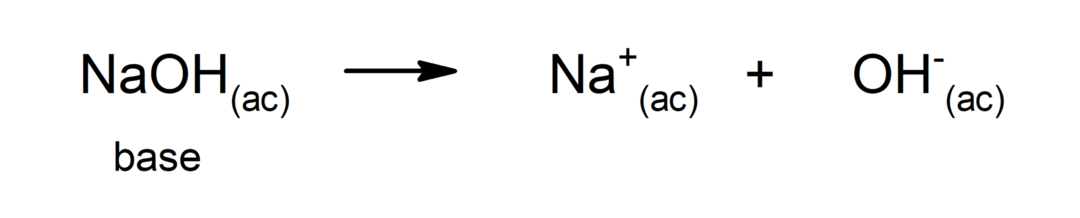
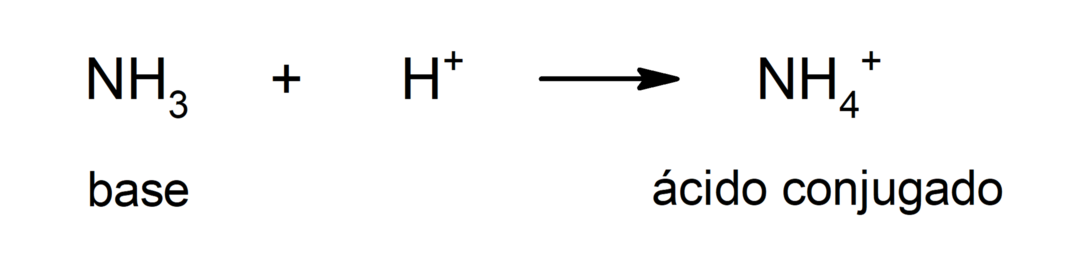

Thus, the OH ion–donates its nonbonding electron pair to the H+to form a coordinate or dative bond (it is a type of covalent bond where only one of the atoms that form the bond provides the pair of electrons) through which the water molecule is formed.
Uses of the bases
The sodium hydroxide It is widely used in the industry: it is the so-called caustic soda. In the manufacture of soap, fats animals or vegetables, which are boiled with hydroxide sodium and thus sodium stearate is formed.
Sodium hydroxide is also used in the manufacture of oven cleaners, in the manufacture of paper pulp, and in some household cleaners. Another widely used base is calcium hydroxide, which is the slaked lime used in construction.
Examples of chemical bases
- Calcium hydroxide - Ca (OH) ₂
- Potassium Hydroxide - KOK
- Barium hydroxide - Ba (OH) ₂
- Magnesium Hydroxide - Mg (OH) ₂
- Ammonia - NH₃
- Copper (II) hydroxide - Cu (OH) ₂
- Iron (II) hydroxide - Fe (OH) ₂
- Aluminum hydroxide - AI (OH) ₃
- Zinc Hydroxide - Zn (OH) ₂
- Sodium hydroxide - NaOH
- Nickel (II) hydroxide - Ni (OH) ₂
- Detergent soap




