Example of Charles's Law
Physics / / November 13, 2021
Charles's gas law or Law of constant pressure, is another of the gas laws, enunciated by Gay-Lussac, who made known the work of Jacques Charles, published around 20 years before.
Charles's law predicts the behavior of a mass of gas when the pressure remains constant and the temperature and volume vary.
Charles's law is stated as follows:
At constant pressure, the volume of a gas is directly proportional to the change in its temperature.
Constant pressure: it refers to the fact that the pressure that the gas exerts on the walls of the container will not vary throughout the experience.
Volume: it is the occupied space that the gas occupies, in general it is considered a container with walls that do not deform, and whose lid works like a plunger.
Temperature: It is the increase or loss of heat that the gas undergoes during the experimentation. If the temperature increases, the volume increases. If the temperature decreases, the volume also decreases.
Algebraically, Charles's Law is expressed with the following formula:

Where:
V = gas volume
T = gas temperature
k = constant of proportionality for that mass of gas.
This means that for a given mass of gas, at constant pressure, the relationship between the volume and the temperature variations, will always have the same proportionality relationship, represented by the constant k:

So once the constant is determined, we can calculate any of the other values from the other known data:
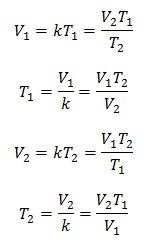
3 Examples of Charles's Law applied to problems:
Example 1: Calculate the new volume, if in a container there is a mass of gas that occupies a volume of 1.3 liters, at a temperature of 280 K. Calculate the volume when reaching a temperature of 303 K.
V1 = 1.3 l.
T1 = 280 K
V2 = ?
T2 = 303 K
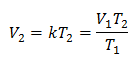
Substituting values:
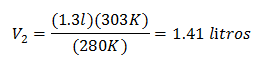
The new volume at 303 K is 1.41 liters.
Example 2. If we have a gas that occupies 2.4 liters at 10 degrees Celsius, calculate the final temperature, if at the end it occupies 2.15 liters.
V1 = 2.4 l
T1 = 10 ° C = 283 K
V2 = 2.15 l
T2 = ?
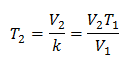
Substituting values:
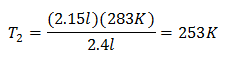
The new temperature is 253 K, which is equal to -20 ° C.
Example 3. We have a gas for which we know that its initial temperature is 328 K, its final volume is 3.75 l, and its ratio constant is 0.00885.
V1 = ?
T1 = 328 K
V2 = 3.75 l
T2 = ?
k = 0.00885
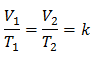
Substituting values:
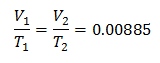
To know the Initial Volume:
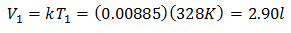
The initial volume is 2.90 l.
To know the final temperature:
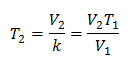
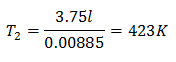
The final temperature will be 423 K, which is equal to 150 ° C.