Definition of Raoult's Law
Miscellanea / / February 21, 2022
concept definition
It is one of the laws of chemistry, developed by the Frenchman Raoult, where it is established that the partial vapor pressure of a component that forms a mixture is equal to the product of the partial pressure of that same pure component by the mole fraction of it in the mixture. mix.

Chemical engineer
Perhaps, it is necessary to redefine the concept of vapor pressure, understanding it as the pressure exerted by the gaseous phase on a liquid phase (both in Balance), at a certain temperature in a closed system. This dynamic equilibrium is reached faster the larger the contact surface between the phases and, in this condition, we speak of saturated phases, both vapor and saturated liquid.
This law laid one of the foundations of the thermodynamics in 1887 and, following the logic From Raoult's Law, we see that the vapor pressure of a substance decreases in value when it goes from being pure to being part of a mixture. Based on the above, the expression The math of it is as follows:
Pi = xiL Pi0
This means that the partial vapor pressure of a substance i in a mixture, P_i, is equal to the vapor pressure of the pure component, Pi0, (at the same temperature) times its mole fraction in the liquid phase, xiL.
When talking about this Law, we refer to typical graphs like this:
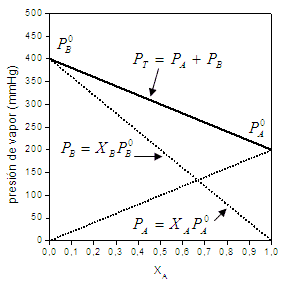
Image taken from UCR3
this simple graphic It is very useful for estimating partial vapor pressures when a substance is part of a mixture, it also allows us to describe the composition of volatile solvents of a mixture, in its gaseous phase as well as many others Applications.
The mathematical expression of Raoult's Law was indicated in each graph for the components of the mixture A and B, in this case, a binary mixture formed by two pure substances. On the abscissa axis we observe the molar fractions corresponding to each component (in liquid phase), having to the left greater amount of component B and, in proportion, lesser amount of component A until the molar fraction of component B is 1 and that of A is 0. While, to the right, the fraction of component A increases, until only component A is obtained (xA=1). On the axis of the ordinates, respectively, we have the vapor pressures of the pure components, that is, when only has component A (xA=1) we have the vapor pressure of this same component and, conversely, it occurs on the ordinate axis of the left. In the transition, the total pressure of the gas phase mixture does not correspond to the vapor pressure of each of the components. but rather to the sum of the partial pressure of its components (Dalton's Law), each of them being estimated from the law of Raoult.
It should be noted that the original Law has modifications based on deviations from the ideality of its compounds. When, depending on the intermolecular forces that come into play, there are interactions between both substances, this generates deviations, since there will be more or less tendency of one of them to remain in phase liquid or not.
In short, when the deviation is negative from Raoult's ideal Law, the intermolecular forces in the solution are greater than in the pure components, therefore, the total pressure will be less than the Estimated. This means that the adhesive forces are stronger than the cohesive forces, which implies that the components are retained in the liquid phase of the mixture by forces of attraction greater than those of pure liquids. On the other hand, if the deviation is positive, the total pressure will be higher than the estimated one since the intermolecular forces in the solution are lower than in the pure components. Here, the cohesive forces between molecules are more important than the adhesive forces, therefore, the components have an easier time passing into the gas phase.
Mainly, Raoult's Law is applied in industrial techniques and to scale laboratory, in processes such as distillation and fractional distillation.
Topics in Raoult's Law

