Concept in Definition ABC
Miscellanea / / April 22, 2022
concept definition
Azeotropes are mixtures of chemical compounds, with a defined composition, that boil at certain temperatures. The fundamental characteristic is that the mixture behaves as if it were a single pure substance, conserving its properties and providing ease of handling.

Chemical engineer
The mixture can be given by two or more components and its composition it is the same both in the liquid phase and in the gas phase, this generates similarities with pure substances. From this, it is easily deduced that this mixture cannot be separated into its components by processes such as fractional distillations since, as mentioned above, it will retain its proportions, behaving as if it were a single component.
Another characteristic is that the boiling point of this mixture can be higher, lower or even equal to that of one of its components. When the azeotrope boils at a temperature of maximum boiling, it is known as maximum azeotrope and when it does so at the lowest possible temperature it is known as minimum azeotrope. This means that the minimum azeotrope has a lower boiling point than its pure components and vice versa with the maximum azeotrope. For example, in the mixture methanol - benzene, the azeotrope is minimum (0.61 benzene - 0.39 methanol in fractions molar) whose boiling point is 58 °C, in a tank where the boiling point of benzene is 80 °C and that of methanol 65°C
Minimum and maximum azeotropes
Now… where does an azeotrope come from? When there is a deviation from the Law Raoult, that is, the vapor pressure of the azeotropic mixture is not directly related to its molar fraction, it is when the concept of azeotropic mixture arises. These deviations occur when the components significantly attract or repel each other, that is, the intermolecular forces are very different in different compounds.
When the deviation is positive, we have the minimum azeotrope, while if the deviation is negative, we have the maximum azeotrope. To better understand this concept, let's look at some graphics:
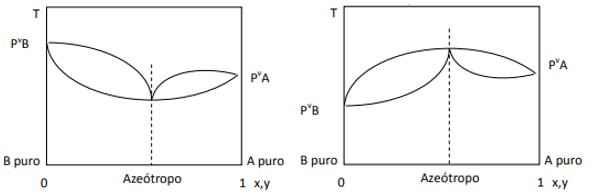
Here we observe that, if the mixture were ideal, by Raoult's Law, the vapor pressures of the pure components should be joined by a straight line, since well, there is a deviation from that law that causes the formation of minimum and maximum azeotropes, depending on whether there is greater or lesser affinity between the components. When the deviation to Raoult's law is negative, as in the figure on the left, it is a minimum at diagram of pressure, but a maximum in the temperature diagram (hence, azeotrope of maximum). On the other hand, if the deviation is positive to Raoult's Law, it will be a maximum in the pressure vs. composition, but a minimum in the diagram of temperature vs. composition (here we have a minimum azeotrope). Do not confuse diagrams pressure vs. composition and temperature vs. composition.
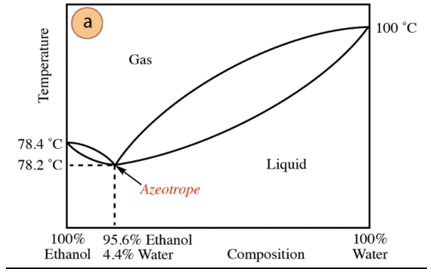
In the case of the ethanol-water mixture, there is a minimum azeotrope whose composition is 95.6% ethanol and 4.4% water. The boiling temperature of the azeotropic mixture is 78.2 °C lower than that of the pure components. So, suppose distilling a water-ethanol mixture with a high water content (close to 100%). It will not be possible to obtain pure alcohol, since larger fractions will be obtained in each distillation stage. of ethanol, but never reaching 100%, it will gradually approach the composition of the azeotropic mixture (the lowest point under). That is why, like water, it has a tendency to form minimum azeotropes with organic compounds (given the type of intermolecular forces that interact), it is required to previously remove the water from the mixture to obtain the pure compound. Or, once the azeotrope is obtained, methods of extraction of additional water.
Based on the above, it is not by chance to find alcohol (in the supermarket and pharmacy) at 95%, is the cheapest product that can be obtained. To obtain pure alcohol, it is necessary to "break" the azeotrope with technique additional, such as the use of drying agents or azeotropic distillations. Azeotropic distillation consists of adding an extra component to the mixture that acts by entrainment and by interaction with any of the components of the mixture (higher affinity) can be removed from the mixture.
Topics in Azeotropes
