Definition of Chemical Equilibrium
Inhibition String Theory / / April 02, 2023
1. Condition of stability present in reversible reactions where the forward and reverse reaction rates always remain the same.
Cat. grammatical: masculine noun
in syllables: e-qui-li-brio + quí-mi-co.
Chemical balance

Chemical engineer
A reaction is in balance chemical when the reaction speed direct is equal to speed reverse reaction. All chemical reaction has a certain spontaneity towards equilibrium, and to investigate it we do it through the sign of ∆G, Energy Gibbs free, which implies that, through the value of this magnitude, we can predict whether a reaction will occur in a certain direction or not.
The variation of the Gibbs Free Energy is expressed, in general, in standard conditions as the difference between the energies of products and reactants also in standard state:
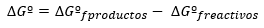
While, if the reaction occurs under non-standard conditions, the relationship between ∆Gº and ∆G is determined by the following expression:
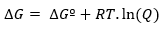
where Q is the reaction quotient.
To understand the implications of the reaction rate and chemical equilibrium we must study the sign of ∆G:
If ∆G is negative, it implies that the reaction is spontaneous (occurs) in the direct sense.
If ∆G is positive, it implies that the reaction is not spontaneous (does not occur) in the direct sense.
While, if ∆G=0, there will be no change, since the system is in equilibrium, and as already mentioned, the direct reaction rate is equal to the indirect reaction rate. This implies that the reaction quotient Q is equal to the equilibrium constant K, so there is no tendency to favor a specific direction of the reaction.
Since Q is defined as:
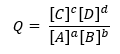
For a generic reaction:
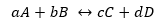
While K takes the same form, but with the concentrations in equilibrium.
If we go back to the case where ∆G is negative, this implies that the reaction quotient Q is less than K (constant of equilibrium), implies that the product concentrations are less than what they should be if the reaction were in progress. balance. Therefore, in terms of spontaneity, it becomes spontaneous in the direct sense.
Whereas, if ∆G is positive, there will be a preponderance of products above what there should be if the system were in equilibrium, with Q being greater than K. Therefore, the reaction is spontaneous in the reverse direction.
It should be noted that the strict definition of Q and K is given in terms of activities of the products and reactants, defining the activity in terms of concentration or pressures as:
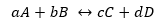
O well:
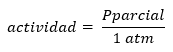
From there it arises that both Q and K are dimensionless and can be considered in both concentrations and partial pressures.
When the concentrations or partial pressures of products and reactants remain constant over time, the situation occurs chemical equilibrium, while a situation of dynamic equilibrium is reached because the rate of forward and reverse reaction with identical. It is important to highlight the dynamism of balance, the speed with which they are formed and consume products and reactants is the same, which is why the concentrations or partial pressures do not it varies.
If the condition moves away from the equilibrium situation, certain species will predominate over others and from there arises the expression that relates the rate of direct and inverse reaction, Kc:
Let's assume the reaction seen above:
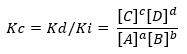
Being Kd and Ki, the reaction rate constants in the forward or reverse direction respectively.
Again, if Kc>1, it implies that Ki is less than Kd, so there is a high degree of conversion of products to reactants. In this case, the equilibrium is shifted towards products.
The reverse occurs if Kc<1, implying that the direct reaction rate is lower than the indirect reaction rate and there is little consumption of reactants, the equilibrium is shifted towards reactants.
Whereas, if Kc=1, the velocities are equal and the system is in equilibrium.
It is important to define two issues: first, the value of this constant depends exclusively on the temperature and, in turn, varies according to the magnitude used to express the concentrations or pressures of products and reactants.
Lastly, the law Chemical equilibrium adjusts for dilute solutions or gases under low pressure.



