What is Electrolysis
Chemistry / / July 04, 2021
In Chemistry, Electrolysis is the phenomenon in which an electric current runs through an aqueous solution of an ionic compound, and starts direct the ions (charged particles) of Compound a two Electrodes, positive (Anode, attracts negatively charged anions) and negative (Cathode, attracts positively charged cations). This phenomenon is governed by the law of Electrostatics, which indicates that opposite charges attract each other.
Electrolytes
In 1883, Michael Faraday he discovered that aqueous solutions of certain substances conduct electric current, while solutions of other substances do not.
To test whether or not an aqueous solution conducts electric current, Faraday designed a simple apparatus consisting of a 110 volt DC circuit, a lamp, Y two metal or graphite electrodes connected to the current source.
If the electrodes are immersed in water, the amount of current flowing is so small that the lamp does not light; the same is true if they are dipped in a sugar solution.
On the contrary, if they are immersed in a solution of
Sodium Chloride NaCl or from Hydrochloric Acid HCl, the lamp shines brightly, which proves that the dissolution is an excellent conductor. On the other hand, using Acetic Acid CH3Concentrated COOH, the solution conducts the current badly, but when the Acid is diluted with Water H2Or, its electrical conductivity increases.During the passage of current through different solutions, different products are obtained at the electrodes.
In the course of his studies on Electrolysis, Faraday deduced the following laws:
1st Law: The amount of substance that has its chemical transformation in an electrode is proportional to the amount of electricity that passes through the solution.
2nd Law: If the same amount of electricity is passed through different solutions, the weights of the substances decomposed or deposited on the different electrodes are proportional to the equivalent weights of said substances.
To cite an example:
It will be assumed that you have five different electrolytic cells. The first with Hydrochloric Acid HCl, the second with Copper Sulfate CuSO4, the third with Antimonious Chloride SbCl3, the fourth with Stannous Chloride SnCl2 and the fifth with Stannic Chloride SnCl4.
The same current is passed through a series of electrolytic cells, until 1,008 grams of Hydrogen have been released (a Equivalent weight of Hydrogen) of the Hydrochloric Acid solution, the weights (in grams) of the other products released in the same time They are:
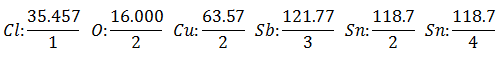
The Equivalent Weight has the value of Atomic Weight of the Element divided by the Valencia of the Element.
To release an equivalent Weight of any item, you need 96500 Coulombs. This amount of electricity is called 1 Faraday.
The Faraday Unit
Ampere is defined as a uniform stream that deposits 0.001118 grams of silver (Ag) from a solution of Silver Nitrate (AgNO3) in a second. Since the Atomic weight of Silver is 107.88 g / mol, the ratio 107.88 / 0.001118 gives the number of Ampere-seconds or Coulombs electricity required to deposit a chemical equivalent of Silver. This quantity is 96494 Coulombs (the 96500 value is fairly approximate for simpler calculations), and is called 1 Faraday of Electricity.
Electrodes
Faraday called Anode to Positive Electrode, and Cathode to Negative Electrode. He also created the terms Anion and Cation, applied to the substances that appear respectively at the anode and at the cathode during Electrolysis.
Currently, another definition for Electrodes is:
Anode: Electrode in which there is loss of electrons or oxidation.
Cathode: Electrode in which there is electron gain or reduction.
Electrolytes and Non-Electrolytes
The conduction of electric current through solutions was not satisfactorily explained until 1887, when Svante Arrhenius made his theory known. Before appreciating and understanding Arrhenius theory, we first set out some of the facts that were known to science when Arrhenius formulated it:
The Non-Electrolyte Solutions they have properties that can be calculated by applying Raoult's law. The vapor pressures and the observed Boiling and Freezing Points of these solutions are practically the same as the calculated values.
The Raoult's Law explains that the Vapor Pressure of each Solute in Solution depends on its own mole fraction in it, multiplied by its Vapor Pressure in its pure state.

Raoult's Law fails when applied to Electrolyte Solutions in Water. The variations of the vapor pressure and of the Boiling and Freezing Points are always greater than those predicted by the aforementioned law, and, furthermore, they increase when diluting.
Such deviations are represented by the value i, which is the ratio of the variation observed in the freezing point between the variation calculated in the freezing point:

The value of i is a measure of the deviation from Raoult's Law, being equal to 1 when there is no deviation.
Electrical conductivity of Electrolytes
Arrhenius investigated the conductivity of aqueous electrolyte solutions to find out how conductivity varied with electrolyte concentration.
It measured the Molar Conductivity (which is the Conductivity corresponding to one mole of dissolved Electrolyte; that is, the specific conductivity referred to one mole, and he found that it increased with dilution.
Arrhenius compared his results with measurements of deviations from Raoult's Law, and found a close relationship between these and molar conductivity. In his theory the behavior of electrolytes is explained:
“The Electrolyte Molecules dissociate into electrically charged particles called ions. The dissolution is incomplete, and there is an equilibrium between the molecules and their ions. The ions conduct the current as they move within the solution ”.
Deviations from Raoult's Law are due to the increase in the number of particles resulting from the partial dissociation of the molecules.
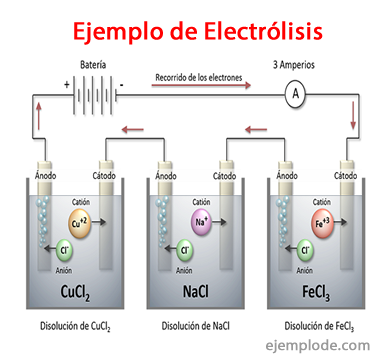
Examples of Electrolysis
Some solutions that behave like Electrolytes, that is, they have the capacity for Electrolysis are:
Sodium Chloride NaCl
Hydrochloric Acid HCl
Sodium Sulfate Na2SW4
Sulfuric Acid H2SW4
Sodium Hydroxide NaOH
Ammonium Hydroxide NH4Oh
Sodium Carbonate Na2CO3
Sodium Bicarbonate NaHCO3
Nitric Acid HNO3
Silver Nitrate AgNO3
Zinc Sulfate ZnSO4


