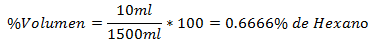Example of Concentration: Molarity, Molality, Normality and Percentage
Chemistry / / July 04, 2021
The Concentration is defined as the Quantity or Proportion of a certain substance, present in a Solution. The Solution is a homogeneous mixture (single phase visible) that can be solid, liquid or gaseous, so Concentration can be expressed in different ways.
It is necessary to define that a Solution contains two main components: Solute and Solvent, and generally, Concentration focuses on expressing how much Solute is mixed in the Whole Solution. However, the Concentration can express the quantity or proportion of any of them.
Expression of Concentration
In Chemistry, the amount of substance present in a solution can be expressed in several different ways: Molarity, Molality, Normality, Percentage by weight, Percentage by volume.
Of these five units, all can be applied to solid, liquid and gaseous solutions. But Molality, for example, is the one that is used the most for solid solutions.
Examples of Molarity
The Molarity indicates how many moles of substance are in each Liter of Complete Solution
. It is the most used unit in chemistry for liquid solutions, when doing Volumetric Analysis. It is indicated by the letter "M".Knowing the grams of Solute, they are divided by the Molecular Weight of the Solute. Thus, the Moles of Solute present in the Solution are obtained.
Then, the Moles of Solute are divided by the Liters of Solution, and thus the units of Molarity are obtained: Moles of Solute / Liter of Dissolution.
1.- For a solution of 0.5 liters of Magnesium Hydroxide [Mg (OH)2], and if the Molecular Weight of Magnesium Hydroxide is 58 g / mol. You have 36 grams of it.
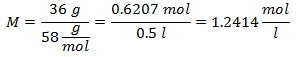
2.- For a 1 liter solution of Calcium Hydroxide [Ca (OH)2], and if the Molecular Weight of Calcium Hydroxide is 74 g / mol. You have 42 grams of it.

3.- For a solution of 3 liters of Sodium Chloride (NaCl), and if the Molecular Weight of Sodium Chloride is 58 g / mol. You have 28 grams of it.
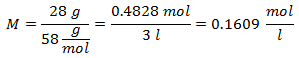
4.- For a solution of 5 liters of Calcium Chloride (CaCl2), and if the Molecular Weight of Calcium Chloride is 110 g / mol. You have 13 grams of it.
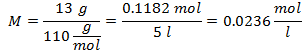
5.- For a solution of 10 liters of Sodium Sulfate (Na2SW4), and if the Molecular Weight of Sodium Sulfate is 142 g / mol. You have 96 grams of it.
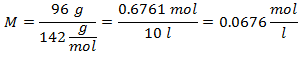
Examples of Molality
The Molality indicates how many moles of Solute there are per 1000 grams of Solvent in the Solution. It is the most widely used unit in chemistry for solid solutions. It is indicated by the letter "m".
Knowing the grams of Solute, they are divided by the Molecular Weight of the Solute. Thus, the Moles of Solute present in the Solution are obtained.
Then, the Moles of Solute are adjusted for each 1000 grams of Solution, which are established as the basis of calculation, and thus the units of molality are obtained: Moles of Solute / 1000g of Solvent
1.- For a solution with 1000g of Mineral solvent and 36 grams of Magnesium Hydroxide [Mg (OH)2], and if the Molecular Weight of Magnesium Hydroxide is 58 g / mol.

2.- For a solution of 2000g of Mineral solvent and 42 grams of Calcium Hydroxide [Ca (OH)2], and if the Molecular Weight of Calcium Hydroxide is 74 g / mol.
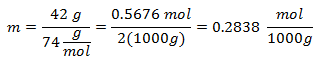
3.- For a solution of 3000g of Mineral solvent and 28 grams of Sodium Chloride (NaCl), and if the Molecular Weight of Sodium Chloride is 58 g / mol.
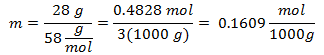
4.- For a solution of 5000g of Mineral solvent and 13 grams of Calcium Chloride (CaCl2), and if the Molecular Weight of Calcium Chloride is 110 g / mol.
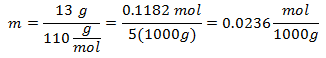
5.- For a solution of 10,000g of Mineral solvent and 96 grams of Sodium Sulfate (Na2SW4), and if the Molecular Weight of Sodium Sulfate is 142 g / mol.
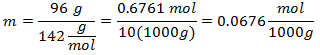
Solvent quantities are handled as multiples of 1000g, to leave 1000 grams as a reference and not include them, affecting the calculation.
Examples of Normality
The Normality indicates how many substance equivalents are in each liter of complete solution. Normality is the other unit most used in chemistry for liquid solutions, when doing Volumetric Analysis. It is indicated by the letter "N".
An Equivalent is a unit that results from dividing the grams of the Solute (g) by its Equivalent Weight (Small). The Equivalent Weight (Peq) results from dividing the Molecular Weight (PM) by the active Valencia (*), which is easier to observe in Acids and Bases. For example, the active Valencia of Hydrochloric Acid (HCl) is 1; the active Valencia of Calcium Hydroxide [Ca (OH)2] is 2, due to the Hydrogen (H +) and Hydroxyl (OH-) ions that each one presents.
Then, the Solute Equivalents are divided by the Solution Liters, and thus the units of Normality are obtained: Solute Equivalents / Solution Liter.
1.- For a solution of 0.5 liters of Magnesium Hydroxide [Mg (OH)2], and if the Molecular Weight of Magnesium Hydroxide is 58 g / mol. You have 36 grams of it.
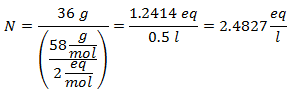
2.- For a 1 liter solution of Calcium Hydroxide [Ca (OH)2], and if the Molecular Weight of Calcium Hydroxide is 74 g / mol. You have 42 grams of it.
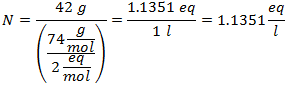
3.- For a solution of 3 liters of Sodium Chloride (NaCl), and if the Molecular Weight of Sodium Chloride is 58 g / mol. You have 28 grams of it.
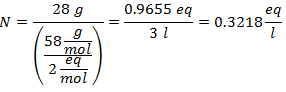
4.- For a solution of 5 liters of Calcium Chloride (CaCl2), and if the Molecular Weight of Calcium Chloride is 110 g / mol. You have 13 grams of it.
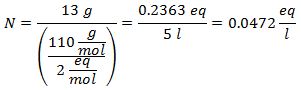
5.- For a solution of 10 liters of Sodium Sulfate (Na2SW4), and if the Molecular Weight of Sodium Sulfate is 142 g / mol. You have 96 grams of it.
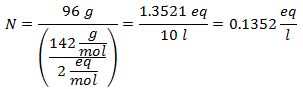
Examples of Percentage by Weight
The Percentage in Weight is an expression of the Concentration that results from dividing the Amount in grams of Solute between grams of Total Solution. Obtaining a decimal quantity, it is multiplied by 100 and is expressed with the symbol "%". This unit is generally used to measure concentrations in solid or granular mixtures.
1.- A mixture of 1300g total contains 13g of Calcium Carbonate. The Percentage in Weight is:
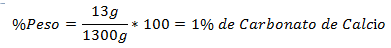
2.- A mixture of 100g total contains 26g of Sugar. The Percentage in Weight is:
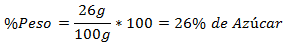
3.- A mixture of 250g total contains 60g of Sodium Chloride. The Percentage in Weight is:
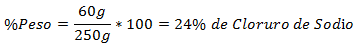
4.- A mixture of 800g total contains 150g of Calcium Hydroxide. The Percentage in Weight is:
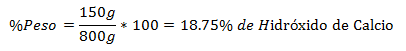
5.- A mixture of 1500g total contains 10g of Sodium Bicarbonate. The Percentage in Weight is:
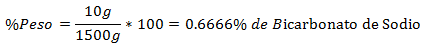
Examples of Volume Percentage
The Percentage in Volume is an expression of the Concentration that results from dividing the quantity in units of Volume of the Solute divided by the Volume of the Total Solution. Obtaining a decimal quantity, it is multiplied by 100 and is expressed with the symbol "%". This unit is generally used to measure concentrations in mixtures of liquid or gaseous components.
1.- A mixture of 1300ml total contains 130ml of Ethanol. The Volume Percentage is:
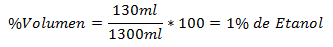
2.- A mixture of 100ml total contains 26ml of Glycerin. The Volume Percentage is:
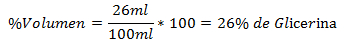
3.- A mixture of 250ml total contains 60ml of Ethyl Ether. The Volume Percentage is:
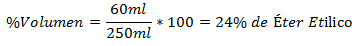
4.- A mixture of 800ml total contains 150ml of Glycol. The Volume Percentage is:
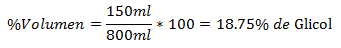
5.- A mixture of 1500ml total contains 10ml of Hexane. The Volume Percentage is: